Fig. 43.1.
Amyloid deposition in the islets of Langerhans.
Mild leukocytic infiltrates
Cystic Fibrosis
♦
Pancreatic involvement with “fibrocystic” appearance in 80–90%
♦
Hyperviscous mucus precipitates in the ducts resulting in duct ectasia
♦
Obstruction-related changes including acinar atrophy and replacement of atrophic lobules by interstitial fibrosis
Inflammatory Conditions
Acute Pancreatitis
Clinical
♦
Most are due to alcohol abuse; some are related to gallstones
♦
Pain and elevated serum enzyme levels (especially amylase)
♦
In some, pain is associated with nausea and vomiting
♦
Variety of other metabolic complications
♦
Local anatomic complications such as pseudocysts (see below), abscesses, hemorrhage, or thrombi
Microscopic
♦
Findings range from “mild edematous pancreatitis” to “severe necrotizing pancreatitis.”
♦
Mild edematous pancreatitis: interstitial edema and usually microscopic fat necrosis, mainly on the surface
♦
Severe necrotizing pancreatitis: extensive and sometimes hemorrhagic tissue necrosis involving both pancreatic parenchyma and extrapancreatic fat tissue, development of complications
♦
Fat necrosis (Fig. 43.2)
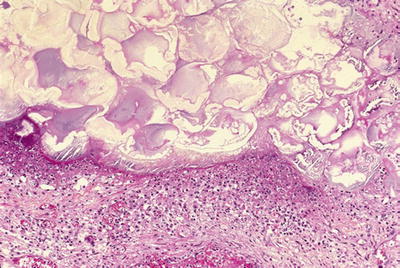

Fig. 43.2.
Acute pancreatitis with fat necrosis. There is a rim of neutrophils around the necrotic focus.
Grossly, chalky white-yellow areas
Fragmented adipocytes with saponification and/or calcification and a rim of neutrophils
Chronic Pancreatitis
Alcohol or Obstruction Related
Clinical
♦
Most are related to alcohol abuse, obstruction (lithiasis or tumors), or other chemical/metabolic factors (hypercalcemia)
♦
Recurrent abdominal pain that is later accompanied by exocrine insufficiency and diabetes
♦
Increased risk of developing a pancreatic carcinoma
♦
Complications such as pseudocyst, persistent stenosis of the bile duct, duodenal stenosis, and fistulas
Macroscopic
♦
Fibrosis and effacement of pancreatic lobular architecture
♦
In some cases, calcifications and intraluminal stones
Microscopic
♦
Ductal dilatation with eosinophilic mucoprotein plugs (with/without calcifications, Fig. 43.3)
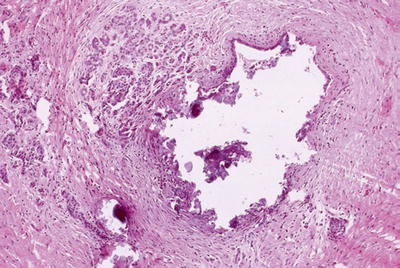

Fig. 43.3.
Alcohol- or obstruction-related chronic pancreatitis (acinar atrophy and interstitial fibrosis) associated with lithiasis represented in this section as calcified intraluminal material.
♦
Remaining small ductules may appear pseudoinfiltrative
♦
Acinar dilatation and atrophy
♦
Interstitial fibrosis with variable cellularity
♦
Remaining islets become prominent and may appear pseudoinfiltrative or form micronodules (Fig. 43.4)
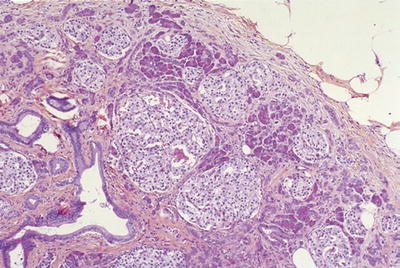

Fig. 43.4.
“Hyperplastic islets.” Islets of Langerhans become prominent or appear proliferative in some pathologic conditions of the pancreas, in particular chronic pancreatitis.
Differential Diagnosis
♦
Ductal carcinoma
Irregularities in the number, spacing, distribution, and contours of the ducts
In some cases, pale, foamy, microvesicular cytoplasm and a dense chromophilic, apical condensation
Cytologic atypia with nuclear enlargement and contour irregularities
Ducts in places that they do not belong: perineurial, intravascular, and in adipose tissue (naked ducts without associated islets or acini)
Autoimmune Pancreatitis, Type 1
Clinical
♦
Typically seen in sixth to seventh decade, male predominance
♦
Slight increase in jaundice compared to autoimmune pancreatitis with granulocytic epithelial lesions
♦
About 25% associated with established autoimmune conditions (PSC, Sjögren, etc.)
♦
Some with “multifocal inflammatory fibrosclerosis” (Riedel thyroiditis, orbital pseudotumor, mediastinal and retroperitoneal fibrosis, etc.)
♦
Radiographically, simulates pancreatic carcinoma; sausage-like appearance of the pancreas and halo formation are characteristic
♦
Elevated serum IgG4 levels (usually >135 mg/dL)
♦
Often responds well to steroid administration
Macroscopic
♦
Localized region of fibrosis and effacement of pancreatic lobular architecture producing mass-like enlargement
♦
Scarring; adhesions to adjacent organs
Microscopic
♦
Dense “duct-centric” inflammation of predominantly lymphoplasmacytic cells and expansion of periductal fibrous tissue (Fig. 43.5)
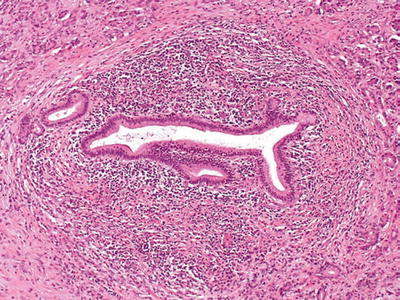

Fig. 43.5.
Type 1 autoimmune pancreatitis. Characteristic periductal lymphoplasmacytic inflammation, with a dense rim of inflammatory cells, and concentric bands of periductal fibrous tissue surrounding the narrowed pancreatic duct.
♦
Periphlebitis and obliterative venulitis are common (Fig. 43.6); frank vasculitis may be seen (not common)
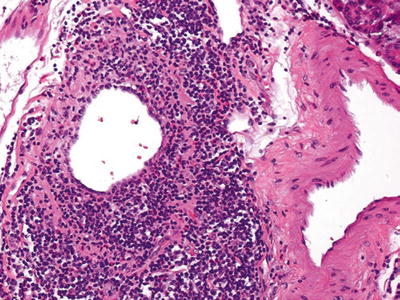

Fig. 43.6.
Type 1 autoimmune pancreatitis. Characteristic venulitis, with aggregates of lymphoid cells in the wall and undermining the epithelium. Sometimes, veins are completely obliterated and replaced by the inflammation and are recognized by their proximity to uninvolved arteries.
♦
Interstitial fibroblastic proliferation with storiform architecture (“inflammatory pseudotumor”-like)
♦
Immunohistologically, >50 IgG4+ plasma cells/HPF is considered highly specific for AIP type 1. However, an elevated IgG4+/IgG+ plasma cell ratio of >40% is more meaningful than IgG4+ plasma cell counts alone in establishing the diagnosis
Autoimmune Pancreatitis, Type 2
Clinical
♦
Seen in fifth to sixth decade, almost equal male/female ratio
♦
Approximately 30% of the cases have associated inflammatory bowel disease, such as ulcerative colitis
♦
It is not associated with serum IgG4 elevation
Microscopic
♦
Intraepithelial neutrophils and occasionally eosinophils (“granulocytic epithelial lesions, GELs”), especially in intralobular ducts, are the hallmark
♦
In the cases with the most severe GELs, the acute inflammatory infiltrate extends into the ductules, acini, and interstitial spaces
♦
Phlebitis is not a common pattern
♦
Has none or very few (<10 cells/HPF) IgG4+ plasma cells
Paraduodenal Pancreatitis
Synonyms
♦
Cystic dystrophy of heterotopic pancreas, groove pancreatitis
Clinical
♦
Predominantly in 40–50-year-old males
♦
History of alcohol abuse is common
♦
Waxing and waning severe upper abdominal pain and postprandial vomiting are common
♦
Predominantly solid ones often mimic pancreas cancer or ampullary/periampullary tumors, radiographically
Macroscopic
♦
Often centered in the region of minor papilla (and the adjacent pancreas)
♦
Scarring of duodenal wall and/or distal common bile duct and/or the “groove” between the duodenum, CBD, and pancreas
♦
Trabeculation of duodenal musculature with occasional cysts (Fig. 43.7)
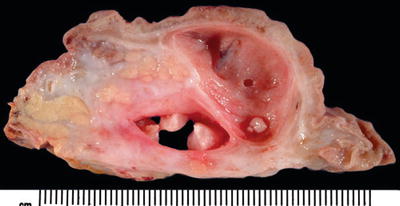

Fig. 43.7.
Paraduodenal pancreatitis. In most cases, there is some degree of thickening and scarring of duodenal wall with trabeculations and associated cysts. Sometimes, one or more cysts achieve large sizes and form paraduodenal wall cyst.
♦
Paraduodenal wall cyst (up to 10 cm) mimicking intestinal duplication may occur
Microscopic
♦
Dense myoid stromal proliferation with intervening rounded lobules of pancreatic tissue (“myoadenomatosis,” Fig. 43.8)
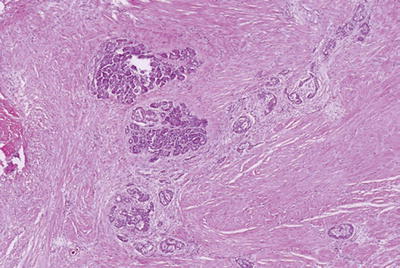

Fig. 43.8.
Paraduodenal pancreatitis. Myoadenomatosis-type changes, which are often associated with accessory duct abnormalities, may form a pseudotumor that can be mistaken clinically as cancer.
♦
Brunner gland hyperplasia
♦
Small cystic spaces lined by cellular stroma
♦
Extravasated (stromal) mucoprotein plugs surrounded by eosinophils or multinucleated giant cells
♦
Prominence of nerve bundles mimicking traumatic neuroma
Pseudocysts
Clinical
♦
Complication of pancreatitis
♦
Most frequently seen in alcoholic males
♦
Most common cystic lesion of the pancreatic region (60–70%) but rarely sampled for pathologic examination
♦
Occurs due to postnecrotic resorption of peripancreatic fat necrosis
Macroscopic
♦
1–15 cm unilocular cyst, filled with necrotic material fluid
♦
Peripancreatic
Microscopic
♦
Depends on the stage of the process
♦
No epithelial lining by definition
♦
The lumen contains necrotic adipose tissue, precipitated acinar secretions, and mucoprotein plugs
♦
The wall consists of dense fibrous tissue with various degrees of granulation-type changes
Differential Diagnosis
♦
Mucinous cystic neoplasm
Epithelial lining
Ovarian-like stroma with more wavy and layered appearance (compared to haphazard cellularity of fibroblastic reaction in pseudocysts)
♦
Other cystic lesions
Epithelial lining or specific characteristic features
Infections
♦
Bacterial, fungal, or parasitic infections may involve the pancreas, but none has any particular affinity for this organ
Neoplasia
Ductal Neoplasia
Invasive Ductal Adenocarcinoma
Synonyms
♦
Pancreatobiliary, tubular
Clinical
♦
>85% of all pancreatic tumors
♦
More common in developed countries, fourth leading cause of cancer deaths in the USA
♦
M/F = 1.5/1, African American/Caucasian = 2/1, mean age 63, very seldom under the age of 40
♦
Proposed risk factors include long-standing diabetes mellitus, smoking, and chronic pancreatitis (especially hereditary pancreatitis); a few are familial, some associated with familial atypical multiple mole/melanoma (FAMMM) syndrome (p16 related)
♦
Common symptoms: abdominal or back pain, weight loss, and jaundice
♦
Some patients develop diabetes mellitus
♦
80% are deemed “unresectable” due to early spread (to mesenteric vessels/retroperitoneum, liver, or peritoneum)
♦
Median survival: 8 months if unresectable but treated with chemo-/radiotherapy and 12 months if resected, 5-year survival <5%; very few patients are alive at 7 years
♦
Almost all develop metastasis (mostly to liver or peritoneum)
Macroscopic
♦
70% occur in the head
♦
Typical size = 3 cm; the tumor is widely disseminated or fatal by the time it reaches 6–7 cm
♦
“Scirrhous” pattern: firm, white-gray or yellow mass with ill-defined borders (Fig. 43.9)
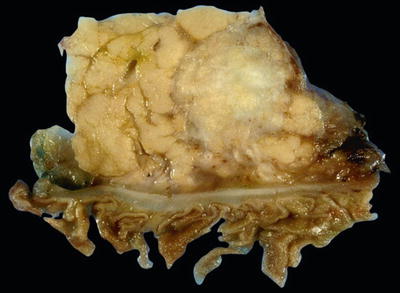

Fig. 43.9.
Ductal adenocarcinoma is grossly a scirrhous carcinoma, characterized by firm white mass with ill-defined irregular borders.
♦
Obstruction of the ducts (in particular, the common bile duct) is typical
♦
May ulcerate into the duodenum
Microscopic
♦
Infiltrating tubular units (often widely scattered with open lumina), cords, or individual cells (Fig. 43.10)
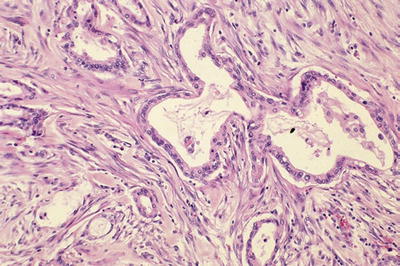

Fig. 43.10.
Invasive ductal carcinomas of the pancreas are characterized by infiltrating small tubular units with open lumina, surrounded by desmoplastic stroma of variable cellularity.
♦
Abundant desmoplastic stroma of variable cellularity
♦
Cuboidal to columnar cells with variable mucin and nuclear atypia
♦
Perineurial invasion in >75% of the cases (Fig. 43.11)
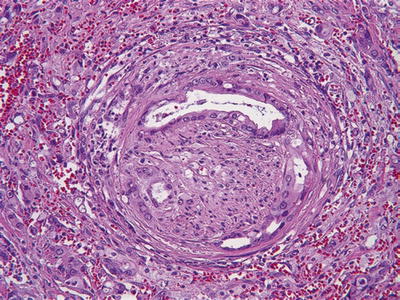

Fig. 43.11.
Perineural invasion is common in invasive ductal carcinoma.
♦
Gland formation and bland cytology may be retained even in perineurial or vascular invasion
♦
Extension to retroperitoneal tissue, especially through the posterior uncinate region, is very common
Variants
♦
Foamy gland pattern (Fig. 43.12)
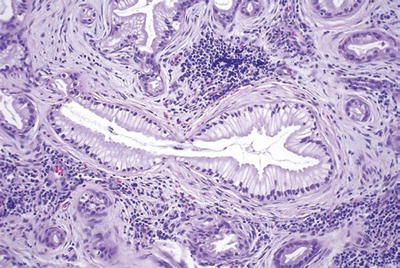

Fig. 43.12.
Invasive ductal adenocarcinoma with foamy gland pattern can be deceptively benign appearing, as reflected in this focus of lymph node metastasis.
Abundant pale, foamy, microvesicular cytoplasm stuffed with small, evenly shaped vesicles
Thin, distinct band of chromophilic condensation in the apical cytoplasm forming a brush border-like zone
♦
Vacuolated cribriform pattern (Fig. 43.13)
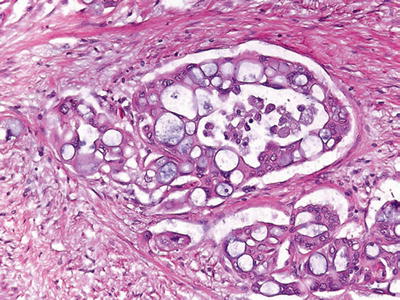

Fig. 43.13.
Cribriform/vacuolated pattern is not uncommon in ductal adenocarcinoma and may help recognize this tumor type in metastatic sites.
Multicell-sized vacuoles resembling lipocytes (or signet ring cells) and forming a cribriform architecture
Vacuoles contain necrotic debris and neutrophils
Nuclear atypia is prominent
♦
Other rare patterns: large duct pattern (mimicking intraductal neoplasia), cord-like formation (Fig. 43.14), clear cell pattern (mimicking renal cell carcinoma), signet ring, hepatoid, oncocytic, and rhabdoid patterns
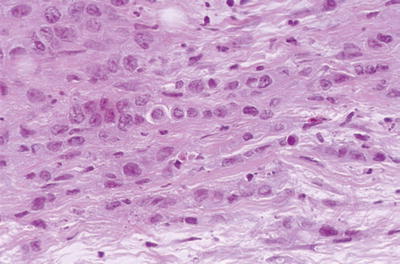

Fig. 43.14.
Invasive ductal carcinoma, cord-like pattern. Often glandular areas are admixed with less differentiated patterns such as this one mimicking mammary lobular carcinoma.
♦
May mimic the primary tumors at metastatic sites
Bronchioloalveolar pattern (“lepidic” growth) in the lung
Mucinous cystic pattern mimicking primary borderline tumors in the ovary
Immunohistochemistry
♦
CK7+, CK20+/−
♦
Mucin positive, especially acidic sialomucin
♦
Mucin-related glycoproteins and oncoproteins [CA19-9, CEA, B72.3, MUC1 (Fig. 43.15), MUC5AC, DUPAN2] +
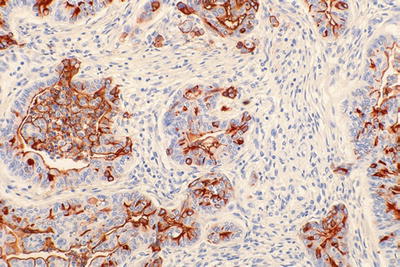

Fig. 43.15.
MUC1 in ductal adenocarcinoma. The labeling is typically apical membranous in the glandular areas and becomes more intracytoplasmic in the less differentiated (nonglandular) component of the tumor.
♦
May contain scattered neuroendocrine cells
♦
Acinar enzymes typically lacking
♦
Loss of DPC4 (SMAD4), seen in approximately 50% of the cases, strongly favors of adenocarcinoma, provided that inbuilt controls are working properly
♦
Alterations in codon 12 of KRAS oncogene (90%), CDKN2A (p16, 95%), TP53 (50%), DPC4 (>50%), BRCA2 (7%), and STK11 (Peutz-Jeghers, 5%) genes
Differential Diagnosis
♦
Noninvasive ducts (benign reactive ducts or intraductal neoplasia)
Smooth contours
Lobular, organized distribution, spatially related to acini and/or islets
Smaller ducts tend to be in clusters; larger ones have elongated lumina
♦
Pseudotumoral pancreatitis
Approximately 5% of pancreatectomies performed with the clinical diagnosis of “pancreas cancer” prove to be chronic pancreatitis (see pancreatitis section)
♦
Ampullary/common bile duct carcinomas
May be morphologically identical
Location is determined by gross examination
Preinvasive neoplasia in the corresponding sites
♦
Nonductal tumors (acinar, neuroendocrine, solid pseudopapillary neoplasia, and pancreatoblastoma)
Stroma poor (no desmoplasia)
Cellular, nonglandular neoplasia with sheetlike or nested growth patterns
Relatively uniform cytology
♦
Intestinal-type adenocarcinoma (gastrointestinal or ampullary origin)
Basophilic appearance, columnar cells, stratification
Large, tubular units with relatively compressed lumina
CK7−, CK20+, MUC1−/+, MUC2+, MUC5AC−
♦
Ovarian adenocarcinoma
Cellular, tubular areas composed of long, branching units with slit-like spaces
Many psammoma bodies
Tufts and micropapillae
WT1+/MUC5AC−
Other Related Carcinomas
♦
Undifferentiated (sarcomatoid, Fig. 43.16): often, ordinary tubular pattern in the background
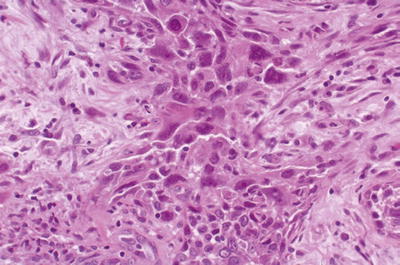

Fig. 43.16.
This undifferentiated carcinoma is composed of large, pleomorphic cells with sarcomatoid transformation.
♦
Adenosquamous and squamous:
Also have dismal prognosis like ordinary ductal adenocarcinomas
Immunohistochemical stains for p63 and CK903 confirm the squamous differentiation
Rare Carcinomas of Presumed Ductal Origin
Colloid Carcinoma
Definition
♦
Invasive carcinoma composed almost exclusively of extracellular mucin pools that contain scanty, detached carcinoma cells
♦
Almost always associated with intestinal-type intraductal papillary mucinous neoplasm
Clinical
♦
Mean age = mid-60s
♦
Thromboembolism in some cases
♦
Pure examples may have a protracted clinical course
Macroscopic
♦
Mean size = 4.5 cm
♦
Relatively well demarcated
♦
Gelatinous
Microscopic (Fig. 43.17)
♦
Well-defined pools of extracellular mucin that contain scanty, detached carcinoma cells
♦
Cells floating in mucin may form strips, signet ring cells, glands, or stellate clusters
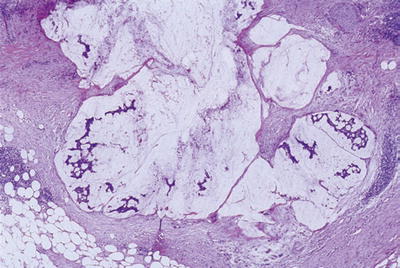
Fig. 43.17.
Colloid carcinoma. Pools of mucin, some of which contain scanty, detached malignant cells.
Differential Diagnosis
♦
Other invasive carcinomas (ductal, signet ring, or others)
Invasive carcinoma cells outside the mucin or at least attached to the stroma
More cells than mucin
Loss of DPC4
♦
In situ neoplasia (mucinous cystic neoplasm, intraductal neoplasia)
Epithelium continuous and well attached to the stroma
Mucin not abundant in histologic sections (washed off during processing)
Undifferentiated Carcinoma with Osteoclast-Like Giant Cells
Clinical
♦
Very rare
♦
Aggressive behavior
Macroscopic
♦
Average size = 10 cm
♦
May appear well demarcated
♦
Most are soft, white-yellow to tan
♦
Some are almost completely cystic or have intraductal growth
Microscopic (Fig. 43.18)
♦
Sea of ovoid to spindle-shaped, highly pleomorphic mononuclear cells, in which a variable number of osteoclast-like giant cells are suspended
♦
Malignant cells are those smaller spindle-shaped, highly pleomorphic mononuclear cells, while the osteoclast-like giant cells are benign
♦
There is usually a conventional ductal adenocarcinoma pattern in the background
♦
Heterologous elements such as bone and cartilage can be seen
Immunohistochemistry
♦
Giant cells are of macrophagic nature; CD45+, CD68+
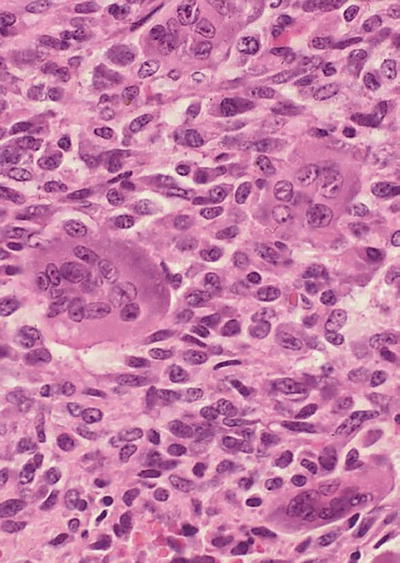
Fig. 43.18.
Undifferentiated carcinoma with abundant osteoclast-like giant cells.
Table 43.1.
Markers of Differentiation/Lineage in Pancreatic Neoplasia
Lineage | Light microscopy | Histochemistry | Immunochemistry | Electron microscopy |
|---|---|---|---|---|
Ductal | Gland, papilla, lumen, mucin | Mucin markers + | Mucin-related glycoproteins and oncoproteins [MUCs, CA19-9, CEA, B72.3 (TAG-72), DUPAN-2] | Mucigen granules, lumina |
Acinar | Sheets of cells, prominent nucleoli, chromophilic (basophilic) cytoplasm, sometimes with granules. Some show acinar pattern | PAS and dPAS+ granules | Enzyme markers (trypsina, chymotrypsin) | Zymogen granules |
Endocrine | Nested, trabecular, gyriform, sheetlike patterns, cytologic uniformity with round nuclei, neuroendocrine-type chromatin | Silver (Grimelius)-positive granules | Neuroendocrine markers (chromogranina, synaptophysin, CD56) | Membrane-bound neurosecretory-type granules |
Medullary Carcinoma
♦
Very rare
♦
Similar to the medullary carcinomas of the breast and colon
♦
Has protracted clinical course
♦
Some have synchronous and metachronous colon carcinomas, and some of these have hereditary nonpolyposis colorectal cancer (HNPCC) syndrome
Microscopic
♦
Pushing border-type infiltration
♦
Syncytial growth pattern
♦
Lymphoplasmacytic infiltrates
Immunohistochemistry
♦
Associated with microsatellite instability (loss of MLH1 and/or MSH2 expression)
Differential Diagnosis
♦
Metastatic carcinomas
♦
Acinar cell carcinoma
Trypsin+, MLH1, and MLSH2+ (not lost)
♦
Poorly differentiated ductal carcinoma
Scirrhous (scar-like) pattern
Small unit infiltration (tubules or cords)
Desmoplasia
Preinvasive Ductal Neoplasia
Pancreatic Intraepithelial Neoplasia
Definition and Classification
♦
Microscopic/incidental forms of preinvasive neoplasia in the ducts
♦
Encompasses a spectrum of changes from those previously called mucinous hypertrophy/metaplasia to frank CIS
♦
Spectrum graded as low-grade PanIN and high-grade PanIN (CIS) (per Baltimore consensus, December 2015)
♦
Presumed precursors of ductal adenocarcinoma
♦
Low-grade PanIN is a common, incidental finding even in normal pancreata
♦
High-grade PanIN is very common in pancreata with invasive ductal carcinoma and is exceedingly uncommon otherwise
Microscopic
♦
Not grossly detectable
♦
Atypical proliferative changes in the native ducts
♦
Ducts involved are typically <0.5 cm
♦
Low-grade PanIN: Simple, columnar, mucin-filled, perfectly polarized cells with small nuclei (previously PanIN-1A) or these changes + mild folding of the epithelium and early (basal) stratification (previously PanIN-1B; Fig. 43.19) or pseudostratification of nuclei; nuclear enlargement, more prominent folding (papillae); some cytologic atypia (previously PanIN-2; Fig. 43.20)
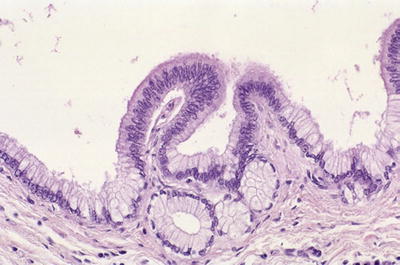
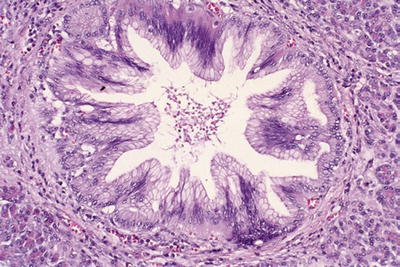

Fig. 43.19.
Low-grade PanIN (Previously PanIN-1). In the background of columnar, mucin-filled, polarized cells (previously PanIN-1A), the mucosal folds at the center show mild stratification of cells (Previously PanIN-1B).

Fig. 43.20.
Another example of low-grade PanIN (Previously PanIN-2). This duct shows papillary folds with stratification of mildly irregular cells. Some of the nuclei are depolarized.
♦
High-grade PanIN: loss of polarity, irregular stratification, tufting, necrosis, mitosis, cytologic atypia (nuclear enlargement, pleomorphism, nuclear irregularities; Fig. 43.21)
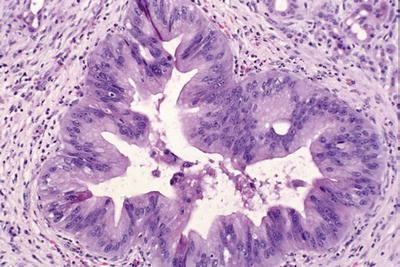

Fig. 43.21.
High-grade PanIN (Previously PanIN-3 or carcinoma in situ). There is loss of polarity, and tuft formation is evident. There are necrotic cells detached into the lumen of the duct. The nuclei are hyperchromatic and irregular.
♦
Clinical significance and rate of progression to invasive carcinoma undetermined
Immunohistochemistry
♦
Mucin of usually acidic sialomucin type
♦
Molecular alterations of ductal carcinogenesis increasing from 1A to 3 (see ductal adenocarcinoma section)
Differential Diagnosis
♦
Intraductal papillary mucinous neoplasms
Mass-forming neoplasia (clinically/grossly detectable papillary or cystic changes)
Size typically >1 cm
Many clinically symptomatic
Tall papillae, some with intestinal phenotype
MUC2 and CDX2 expression, common
♦
Invasive ductal carcinoma
Contour irregularities
Irregular clustering and haphazard distribution
Intraductal Papillary Mucinous Neoplasms (Table 43.2)
Table 43.2.
Differential Diagnosis of Preinvasive Ductal Neoplasia (Intraductal and Cystic Neoplasia With/Without Papilla Formation)
Intraductal papillary mucinous neoplasm | Mucinous cystic neoplasm | |
|---|---|---|
Mean age | 68 | 48 |
Gender | Males > females | Females (>90%) |
Specific endoscopic finding | Mucin extrusion from ampulla | None |
Specific radiologic finding | Cystically dilated pancreatic ducts | Multilocular cyst in the tail of the pancreas |
Preferential location | Head (>80%) | Body or tail (>90%) |
Ductal communication | Yes | No |
Degree of papilla | Prominent | Variable |
Pattern of papilla | Intestinal, pancreatobiliary, gastric | Variable |
Specific histologic findings | Cystically dilated native ducts with villous nodules
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|