(1)
Canberra, ACT, Australia
Summary
Patients can present with pain of odontogenic or non-odontogenic origin, arising from the facial area, temporomandibular joints, ear, eyes, pharynx and larynx. Often they may be complaining of pain from their teeth. The dilemma the clinician faces is finding the correct origin of this pain whereby an effective treatment will provide relief. Patients with neuropathic orofacial pain may present to the clinician with a persistent, severe pain resulting in multiple endodontic procedures being instigated or worse still extractions recommended with no resolution of symptoms. The literature has demonstrated that the incidence of pain following endodontic treatment is between 3 and 6 % in the absence of reliable clinical and/or radiographic evidence of persisting disease. Management of orofacial pain is best by a multidisciplinary approach, and often the dentist is involved from the very beginning. Where patient toothache symptoms are not reproducible or clinical signs do not correlate, then a referral to an endodontist should be sought.
Clinical Relevance
The clinician must be able to differentiate and accurately diagnose endodontic disease associated with both symptomatic and asymptomatic teeth. A complete history recalled and recounted by the patient combined with a careful clinical and radiographic examination is the first step in establishing a differential diagnosis. The clinician must also have an understanding of the pathways and mechanisms, possible causes and different characters of orofacial pain to be able to narrow this diagnosis to the probable cause. Once odontogenic pain is excluded, other potential causes of orofacial pain should be taken into consideration in order to arrive at the likely diagnosis.
1.1 Overview of Orofacial Pain
The international association for the study of pain defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described as such damage’ [1]. Orofacial pain is defined as a term referring to oral pain, dental pain and pain in the face above the neck, anterior to the ears and below the orbitomeatal line [2]. Although odontogenic (dental) pain is the most commonly reported form of orofacial pain when presenting to the dentist, it should be recognised that this symptom could be caused by painful disorders of non-odontogenic origin. Tables 1.1 and 1.2 and Fig. 1.1 provide a comprehensive list of possible diagnoses that need to be considered when dealing with a patient with orofacial pain. Epidemiological studies show that the prevalence of orofacial pain in the population is between 14 and 19 % [3, 4]. A cross-sectional population study revealed that in the general population the prevalence of muscular-ligamentous/soft tissue type of orofacial pain was 7 %, dentoalveolar 7 % and neurological/vascular 6 % [5].
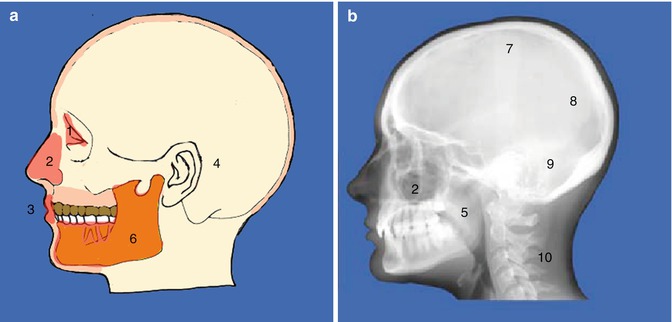
Table 1.1
Differential diagnosis for pain of dental origin
Origin | Possible causes |
|---|---|
Pulpal pain | Dentine hypersensitivity |
Dental caries | |
Defective restoration | |
Cracked tooth syndrome | |
Reversible pulpitis | |
Irreversible pulpitis | |
Pulp necrosis (partial) | |
Periodontal pain | Traumatic periodontitis |
Perio-endo lesion | |
Endo-perio lesion | |
Combined endo-perio lesion | |
Periodontal abscess | |
Gingival pain | Gingivitis |
Acute necrotizing ulcerative gingivitis | |
Pericoronitis | |
Lichen planus | |
Mucous membrane pemphigoid | |
Bone pain | Acute alveolar osteitis (dry socket) |
Table 1.2
Differential diagnosis for pain of non-dental origin
Origin | Possible causes |
|---|---|
Neurologic | Trigeminal neuralgia |
Glossopharyngeal neuralgia | |
Post-herpetic neuralgia | |
Bell’s palsy | |
Vascular | Migraine |
Cluster headaches | |
Giant cell arteritis | |
SUNCT | |
Maxillary antrum | Maxillary sinusitis |
Salivary glands | Acute/chronic bacterial sialadenitis |
Sjogren’s syndrome | |
Calculi causing duct stenosis or obstruction | |
Soft tissue/oral mucosa | Herpes zoster |
Herpetic gingivostomatitis | |
Mucosal ulceration | |
Masticatory muscles/jaws | Temporomandibular joint disorders |
Ears/eyes/nose/lymph nodes | Otitis media |
Glaucoma | |
Psychogenic | Chronic idiopathic facial pain |
Atypical odontalgia | |
Burning mouth syndrome |

Fig. 1.1
(a) Diagrammatic representation and (b) lateral skull x-ray demonstrating local and general causes of orofacial pain. (1) Eyes, (2) nose and sinuses, (3) oral, (4) ears, (5) pharynx, (6) jaws and temporomandibular joints, (7) psychogenic, (8) vascular, (9) neurological and (10) referred pain from other sites. There are a multitude of non-odontogenic pains that can present at the site of a tooth and can mimic a toothache. As a clinician, we should also have an understanding of the complex mechanism of odontogenic pain and the manner in which other orofacial structures may simulate dental pain
Pain management begins with developing an accurate differential diagnosis of dental pain based on clinical signs and symptoms, special tests and radiographic findings. This is the critical first step in pain management and the clinical decision-making process allowing for an effective treatment that is directed at treating the underlying disorder [6, 7]. A detailed history of the patient is a prerequisite to establish a proper diagnosis (see Chap. 11).
The sensory innervation of teeth, terminating in the pulp–dentine complex, is made up of predominantly A beta, A delta and C fibres. The A beta fibres are sensitive to mechanical (hydrodynamic) stimulation of dentine. Myelinated A delta fibres, containing the neuropeptide calcitonin gene-related peptide, are responsible for strong, sharp, immediate well-localised pain. Nonmyelinated, slow conducting C fibres, responsive to inflammatory mediators, are responsible for dull, continuous and irradiating pain [8–10].
The hydrodynamic theory is the most acceptable theory of pain transmission of pain stimuli through the dentine [9]. According to this theory, pain provoked by stimuli is a consequence of fluid flow in the dentinal tubules, stimulating mechanoreceptors (A beta fibres) leading to nerve impulses in the sub-odontoblastic plexus of Raschkow resulting in pain. The effect of thermal stimulus (hot or cold) is explained by the hydrodynamic theory in the following way; application of hot stimuli on the exposed dentine leads to expansion of fluid, whereas cold application causes its contraction. This fluid flow results in activation of mechanoreceptors of the sensory nerves. Chemical stimuli applied (sweet and salty foods) also lead to a faster flow of dentinal fluid to the surface of teeth. This is due to the low concentration of dentinal fluid and due to its lower osmolarity causing fluid flow towards a higher concentration of liquids [11–13].
We can classify pulpal disease according to several conditions: a healthy pulp; an inflamed pulp, which has the capability of reversal (reversible pulpitis); an inflamed pulp without the possibility of recovery (irreversible pulpitis); and pulp necrosis [14, 15].
Dentine hypersensitivity is a direct consequence of exposed dentine reacting to thermal osmotic, chemical and tactile stimuli. The diagnosis is made on symptoms eliciting a sharp pain of short duration in the absence of any pulpal pathology (based on clinical and radiographic signs). Risk factors that contribute to developing dentinal sensitivity include erosion, abrasion, attrition, gingival recession, previous periodontal treatment and anatomical defects. Management strategies involve therapies aimed to reduce dentinal fluid flow (resin application to exposed dentinal tubules) or reduce dentinal neuron activity (use of desensitising agents such as potassium nitrate, strontium chloride, fluoride containing medicaments and guanethidine) [16–21].
Reversible pulpitis indicates that the pulp is vital with some areas of inflammation that has the potential to heal provided the stimulus/irritant has been removed. Symptoms can range from none at all to intense sharp sensations often associated with thermal stimulus [22].
In irreversible pulpitis, the pulp, although vital, is severely inflamed and healing is an unlikely event when steps are taken to try and conservatively manage the pulp [23]. Symptoms vary greatly with some patients complaining of moderate to severe pain exacerbated with thermal stimulus whilst others remain asymptomatic. Indeed pulpitis may proceed to pulpal necrosis without any symptoms at all [24]. Studies have shown that there is a poor correlation between clinical pain symptoms and the histopathological status of the pulp [25].
Finally when the inflammation from the pulp reaches the peri-radicular tissues, the patient is usually able to locate the tooth responsible (symptomatic periodontitis) and/or the clinician is able to demonstrate by clinical and radiographic examinations. Allodynia (a reduction in pain threshold whereby a normal non-noxious stimulus is perceived as pain) and hyperalgesia (an increase in the magnitude of pain perception, so that a previously painful stimulus is now perceived as having a larger magnitude of perceived pain) are symptoms of endodontic disease. Inflammatory mediators are responsible for activation and sensitisation of nociceptors (a sensory receptor that is capable of transducing and encoding noxious stimuli) both peripherally and centrally. This phenomenon can explain why some patients experience pain postoperatively and is an indicator that patients who experience preoperative pain are more likely peri-operatively and post treatment. Patients will often report mechanical and thermal (heat or cold) hyperalgesia and mechanical allodynia in relation to pulpal symptoms preoperatively. Examples include percussion tenderness to a mirror handle that is not painful when demonstrated on a control tooth but obviously painful with a tooth that has pathology of endodontic origin. Clinical pain is not simply the consequence of a ‘switching on’ of the ‘pain system’ in the periphery by a particular pathology, but instead reflects the state of excitability of central nociceptive circuits whose sensitivity can be shifted by normal innocuous inputs [26–28].
In referred pain, the region of the body where the pain is perceived is not the same as where the pain originates. Clinically this type of pain is a diagnostic challenge since effective treatment needs to be directed at the cause, not the site. The reasons behind referred pain are due to central sensitisation and convergence of primary afferent nerve fibres onto the same projection neurons [29]. Afferent sensory nerve neurons have peripheral terminals that innervate different tissues, yet their central terminals converge onto the same second-order projection neuron located in the trigeminal nuclear complex. Examples in endodontics include scenarios where the patient is experiencing pulpitis on the right side and believes it is coming from the maxilla when it in fact originates in the mandible. Another example is when there is maxillary sinusitis and pain is referred to the maxillary teeth on the affected side. The condition of referred pain is a diagnostic challenge whereby pain of non-odontogenic origin can result in radiation of pain to distant sites such as teeth. A typical example is a patient suffering from myofacial pain who often complains of a dull ache in their mandibular posterior teeth. There are a multitude of non-odontogenic pains that can present at the site of a tooth and can mimic a toothache. Dental practitioners should have an understanding of the complex mechanism of odontogenic pain and the manner in which other orofacial structures may simulate dental pain. Failure to establish the aetiology of the pain will result in incorrect diagnosis and inappropriate treatment [30].
The International Association for the Study of Pain (IASP) defines acute maxillary sinusitis as ‘constant burning pain with zygomatic and dental tenderness from the inflammation of the maxillary sinus’ [1]. In chronic cases, there may be no pain or just occasional mild diffuse discomfort. Aetiology is either bacterial or the more common form due to allergies. The pain can be triggered by bending forward, touching the area or pain on biting associated with the upper teeth. Headache is located over the antral area. Diagnosis can be confirmed by maxillary sinus imaging revealing fluid accumulation within the affected sinus. Periapical periodontitis may result in maxillary sinusitis of dental origin with resultant thickening and inflammation of the mucosal lining of the sinus in areas adjacent to the involved teeth. In cases of sinusitis of dental origin, conventional endodontic treatment or re-treatment is the treatment of choice [31]. Antibiotic therapy and the use of nasal decongestants and analgesics are indicated for the treatment of sinusitis or where the sinus has been breached from nonsurgical or surgical endodontics [32].
Temporomandibular disorder (TMD) is an umbrella term associated with myofacial pain, mandibular dysfunction, facial arthromyalgia and masticatory myalgia. It encompasses pain related to the masticatory muscles, the temporomandibular joint (TMJ) or both. Risk factors include female gender, depression and multiple pain conditions often suffering from chronic pain [33]. The relationship between TMD pain and clenching habit or bruxism is controversial. One systematic review showed no association between malocclusion, functional occlusion and TMD in a community-based population [34]. Management strategies include conservative therapies such as provision of information and reassurance, psychological strategies, behavioural changes, posture training, thermal application and mechanical exercises. Stabilisation splint therapy, which is worn at night, prevents clenching and/or grinding (parafunctional habits). Some appliances are designed to realign the maxilla–mandible relationship. The devices are made of either soft plastic or hard acrylic. Evidence for their efficacy has not yet been proven. A number of medical therapies involving a range of drugs from analgesics to antidepressants are also available. Surgical procedures including arthrocentesis and arthroscopy have been advocated when there are functional signs [35].
Trigeminal neuralgia (TN) is defined by the International Association for the Study of Pain (ISAP) as a ‘sudden and usually unilateral severe brief stabbing recurrent pain in the distribution of one or more branches of the fifth cranial nerve’ [1]. Trigeminal neuralgia presents with sharp, shooting, unbearable pain in the distribution of one or more branches of the trigeminal nerve, of moderate to intense severity, lasting seconds. It is precipitated by light touch, but may be spontaneous, and there are often associated trigger points. These trigger points often present around the teeth resulting in irreversible dental procedures carried out unnecessarily [36]. Patients may have periods of remission lasting days, weeks or longer. Symptomatic causes of trigeminal neuralgia need to be excluded such as tumours, multiple sclerosis and arteriovenous malformations [37]. Management includes medical with carbamazepine or surgery (microvascular decompression, ablative procedures and Gamma Knife) [37–39].
Atypical odontalgia clinically can present with persistent pain, often commencing in conjunction with some form of invasive dental treatment such as root canal therapy, apicectomies or history of multiple extractions. This typical presentation with no change in pain and no obvious cause should alert the clinician to a neuropathic cause. The pain is described as being dull and throbbing in nature, often continuous, which can be sharp at times. It can be light touch provoked with features of allodynia at the pain site. No clinical, laboratory or radiographic findings demonstrate relevant abnormality [40, 41].
Chronic (persistent) idiopathic facial pain (CIFP), previously atypical facial pain, is persistent facial pain, which is poorly understood. The diagnosis is often made when all other causes of facial pain have been excluded. The pain is described as aching, dull, nagging, sometimes throbbing, sharp or stabbing. The pain is often constant (mild to severe) with varying intensity but not unbearable. The site of pain varies often not following a neurological distribution. All investigations are normal and patients are likely to be suffering from other chronic pain conditions elsewhere. A multidisciplinary approach has been recommended using a combination of drugs (antidepressants) and cognitive-behavioural therapies [42, 43].
A recent systematic review found the prevalence of persistent pain after endodontic treatment to be 5.3 %. Non-odontogenic pain is not an uncommon outcome after root canal therapy and may represent half of all cases of persistent tooth pain. These findings have implications for the diagnosis and treatment of painful teeth that were previously root canal treated because therapy directed at the tooth in question would not be expected to resolve non-odontogenic pain [44, 45].
Orofacial pain, its diagnosis and initial management fall between dentists and doctors and in the secondary care sector among pain physicians, headache neurologists and oral physicians. Management of neuropathic pain is by accepted pharmacotherapy with psychological support. Facial pain patients should be managed in a multidisciplinary team [46].
Clinicians should always remember the old saying ‘if you hear hoof beats, think of horses, not zebras’. When a patient presents with a disease, good diagnostic judgement should be based on the most frequent cause of this pain, not the obscure, seldom seen disease. A logical diagnosis can only be achieved through careful history taking and clinical assessment incorporating diagnostic tests that will either confirm or disprove your initial thoughts. As dentists we are very good at diagnosing dental pain but should be careful when contemplating irreversible treatments in a patient based on symptomology without clinical signs and radiographic findings which would validate a correct course of action [47].
1.2 Odontogenic Pain
Odontogenic pain can originate from either pulpal or periodontal tissue (see Fig. 1.2). Although the mechanisms for pulpal and periodontal pain are of inflammatory in origin, each pain is perceived differently. Periodontal pain is often well localised (due to proprioceptors present in the periodontal ligament) and the patient can usually point to the tooth, whereas pulpal pain is poorly localised and may be referred to another tooth (on the same side of the arch or the opposite arch on the same side) or another region of the jaw or face (sinuses, angle/ramus of mandible, ears, etc.). Pulpitis can be divided into two categories: reversible and irreversible.
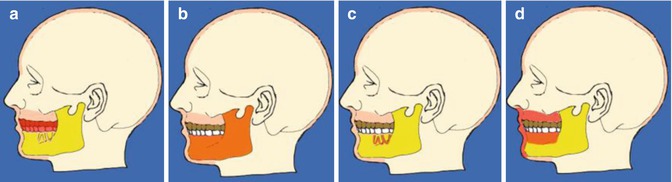

Fig. 1.2
Diagrams showing the common causes of odontogenic pain. (a) Teeth, (b) bone, (c) periodontal and (d) soft tissues. Common features of odontogenic pain include the presence of aetiologic factors of an odontogenic origin (e.g. caries, leakage of restorations, trauma, fracture, periodontal disease, bone infection, soft tissue lesion)
Reversible and irreversible pulpitis
Reversible pulpitis indicates that pulpal tissues can repair once the noxious irritant has been removed. It is often characterised by a short, sharp pain upon provocation and does not occur spontaneously. Irreversible pulpitis has a prolonged duration of pain when stimulated but may also occur spontaneously. There is not usually sensitivity to percussion until the inflammatory process has reached the peri-apex of the tooth).
Necrotic pulp
A necrotic pulp can be completely asymptomatic or extremely painful. The tooth will not usually respond to thermal stimulus such as cold or vitality pulp testing (electric pulp testing). Occasionally sensitivity testing may give a false positive result in multi-rooted teeth where one canal is diseased whilst the others remain vital. If the inflammation has spread to the peri-apex of the tooth, then extreme sensitivity to percussion can occur.
Periodontal pain may occur as a result of endodontic disease (primary endodontic), periodontal disease (primary periodontal) or a combination of both.
Acute apical periodontitis
Inflammation, as a direct consequence to irreversible pulpitis or a necrotic pulp, localises in the apical periodontal ligament. The tooth becomes sensitive to percussion and/or biting and may be mobile.
Acute apical abscess
In this situation the inflammation, often a progression from a necrotic canal or sequelae to irreversible pulpitis, can result in spreading of bacteria into the surrounding alveolar bone (osteitis) and beyond into the fascial planes of the soft tissues (cellulitis). The host defence attempts to counteract this foreign invasion by the release of polymorphonuclear leukocytes resulting in destruction of bacteria and formation of pus. Pain associated with an abscess will be intense due to a build-up of pus and confinement to bone resulting in pain as the pressure increases. The abscess will try to find the pathway of least resistance as the pressure continues to intensify as more and more dead bacteria and host cells collect at the zone of defence between bacteria and host. Occasionally due to extensive bone loss, the cortical plate is no longer intact allowing the abscess to escape through a fistula or sinus. Sometimes the abscess may drain through a narrow pocket beside the tooth. Often we can provide effective relief for the patient by incision and drainage when the abscess is a fluctuant swelling beneath the underlying mucosa. The cardinal signs of acute inflammation such as pain, swelling, heat and redness accompany an abscess as a result of the inflammatory process.
Acute periodontal abscess
In this situation, the acute infection is a direct result of an abscess within the soft tissue wall of an existing periodontal pocket. The abscess will often be well localised with moderate accompanying pain and throbbing. The area may result in a localised swelling as the abscess develops resulting in fluid accumulation within the periodontal ligament space causing the offending tooth site to become more sensitive when chewing/biting and with increased mobility. The overlying gingival tissues will appear swollen, and the patient may have accompanying fever and lymphadenopathy. Management of a periodontal abscess is by providing effective drainage and nonsurgical debridement of the pocket with curettes and irrigation. Systemic antibiotics are not usually indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


