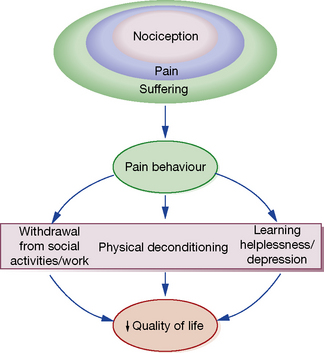
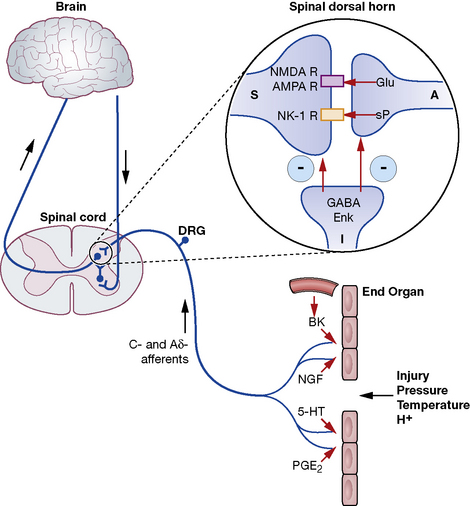
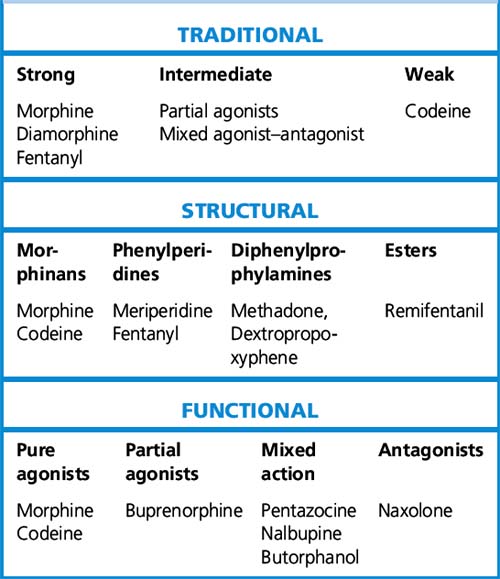
Michael C. Lee, Mark Abrahams The International Association for the Study of Pain defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’. This implies that the degree of pain experienced by the patient may be unrelated to the extent of underlying tissue damage, and that emotional or spiritual distress can add to the patient’s experience of pain (Fig. 18.1). This chapter focuses on the use of drugs for pain relief and illustrates the use of many analgesics that may be encountered in clinical practice. However, clinicians should recognise that the experience of pain is influenced by physical, emotional and psychological factors. While drug therapy is an expedient (and familiar) form of treatment, successful management of pain requires a more holistic approach that addresses all the components of pain.2 Pain alerts us to ongoing or potential tissue damage and the ability to sense pain is vital to our survival. The physiological process by which pain is perceived is known as nociception. While the neurobiology of nociception is complex, its appreciation provides a useful framework for understanding the way analgesics work (Fig. 18.2). Fig. 18.2 Schematic representation of nociceptive pathways. Noxious stimuli such as protons (H +), temperature (temp) etc. applied to end-organs activate nociceptors. Injury leads to the release of prostaglandins such as prostaglandin E2 (PGE2), serotonin (5-HT), nerve growth factor (NGF) etc. from damaged cells, bradykinin (BK) from blood vessels and substance P (sP) from nociceptors. These agents either activate nociceptors directly or sensitise them to subsequent stimuli by parallel activation of intracellular kinases by G-protein-coupled receptors and tyrosine kinase receptors. Primary nociceptive afferents (C-fibres, Ad-fibres) of dorsal root ganglion (DRG) neurones synapse on second order neurones (S) in the spinal dorsal horn (magnified in inset). Here, glutamate (Glu) and sP released from primary afferent terminals (A) activate glutamate receptors (NMDA R, AMPA R, mGluRs) and neurokinin-1 (NK-1) receptors, respectively, located postsynaptically on spinal neurones. These synapses are negatively modulated by spinal inhibitory interneurones (I), which employ enkephalins (Enk) or γ-aminobutyric acid (GABA) as neurotransmitters. Spinal neurones convey nociceptive information to the brain and brainstem. Activation of descending noradrenergic/norepinergic and/or serotonergic systems, which originate in the brain and brainstem, leads to the activation of spinal inhibitory interneurones (I) thereby resulting in antinociception (http://encref.springer.de/mp/0002.htm). • Allodynia – pain due to a stimulus which does not normally provoke pain. • Hyperalgesia – an increased response to a stimulus which is normally painful. • Paraesthesia – abnormal sensation, e.g. ‘pins and needles’. • Dyaesthesia – a painful paraesthesia, e.g. burning foot pain in diabetic neuropathy. Prostanoid is a major sensitiser that is produced at the site of tissue injury. NSAIDs act by inhibiting cyclo-oxygenase, an enzyme involved in the production of prostanoid, as well as other prostaglandins. This enzyme has a number of isoforms, the most studied being cyclo-oxgenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2). Their actions are inhibited by NSAIDs (see Ch. 16). Increased COX-2 production is induced by tissue injury and accounts for the efficacy of COX-2-specific inhibitor drugs (COXIBs). The inhibitory effect of NSAIDs on the production of other prostaglandins is responsible for the common side-effects of these drugs. Among other functions, the prostaglandins produced by cyclo-oxygenase act to protect the gastric mucosa, maintain normal blood flow in the kidney and preserve normal platelet function. Inhibition of prostaglandin production, therefore, can cause gastric irritation, damage to the kidney and an increased risk of bleeding. All NSAIDs are associated with dose-dependent side-effects. In particular, there is a risk of gastrointestinal bleed, renal toxicity and a possibility of cardiac-related complications. The morbidity related to gastrointestinal adverse effects is considerable (~ 5 per 1000 patients per year of treatment) and catastrophic bleeding can occur without any preceding warning symptoms. For this reason, it is recommended that, when used in the longer term, NSAIDs should be prescribed along with an appropriate gastro-protective agent (see p. 531). Opioids have been traditionally classified as strong, intermediate and weak, according to their perceived analgesic properties and propensity for addiction. This approach can be misleading, as it implies that weak opioids such as codeine are less effective but safe. Codeine may be less potent than morphine but can cause respiratory depression if given in sufficient quantities. Codeine-like drugs are also frequently abused. Opioids may also be classified according to their structure. As described later, the properties of opioids may be predicted on the basis of activity on opioid and other receptor systems. The functional classification is probably of most clinical use (Table 18.1). Table 18.1 Classification of opioids
Pain and analgesics
Definition of pain
Nociception
Evaluation of pain
Non-opioid analgesics
NSAIDs (non-steroidal anti-inflammatory drugs)
Mechanism of analgesia
Side-effects
Opioid analgesics
Classification of opioid drugs
Pain and analgesics