9 Pain and anaesthesia
Basic concepts
Pain perception
Activation of nociceptors in the peripheral tissues
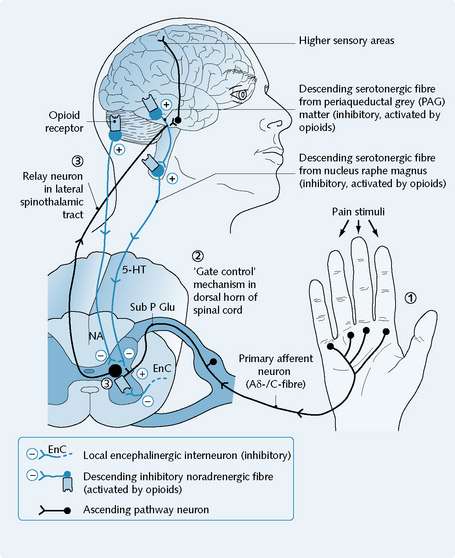
Noxious thermal, chemical or mechanical stimuli can trigger the firing of primary afferent fibres (type C/Aδ), through the activation of nociceptors (pain-specific receptors) in the peripheral tissues (Fig. 9.2).
Transmission of pain information
Onward passage of pain information
The onward passage of pain information is via the spinothalamic tract, to the higher centres of the brain. The higher centres of the brain coordinate the cognitive and emotional aspects of pain and control appropriate reactions. Opioid peptide release in both the spinal cord and the brainstem can reduce the activity of the dorsal horn relay neurons and cause analgesia (Fig. 9.2).
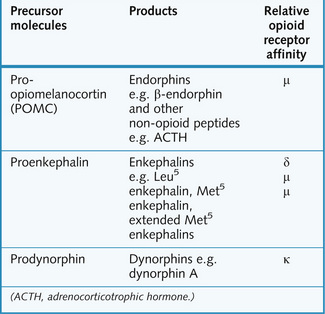
Opioid receptors
Opioid receptor activation has an inhibitory effect on synapses in the central nervous system (CNS) and in the gut (Fig. 9.3).
Secondary-messenger systems associated with opioid receptor activity include:
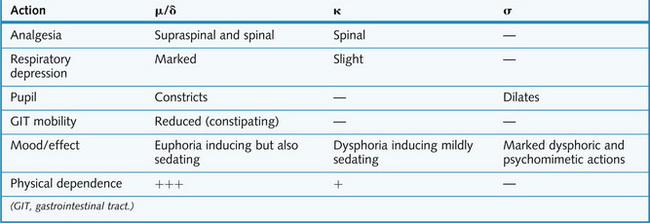
Activation of all opioid receptors by endogenous or exogenous opioids results in:
Opioid analgesic drugs
Route of administration
Oral, rectal, intravenous, intramuscular, transdermal and transmucosal (as lozenges).
Indications
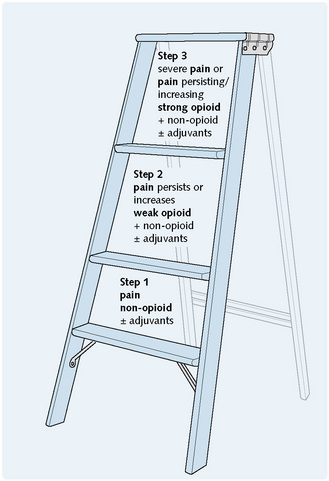
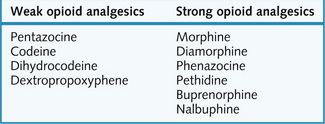
Strong opioids (Fig. 9.5) are used in moderate to severe pain, particularly visceral, postoperative or cancer-related; in myocardial infarction and acute pulmonary oedema; and in perioperative analgesia (p. 146).
Weak opioids (Fig. 9.5) are used in the relief of ‘mild to moderate’ pain, as antitussives (Ch. 3) and as antidiarrhoeal agents (Ch. 8), taking advantage of these ‘side-effects’ of opioid analgesics.
Adverse effects
Central adverse actions include the following:
Peripheral adverse actions include the following:
Constipation and nausea are also common problems and clinically it is common to co-administer laxatives and an antiemetic (Ch. 8).
Tolerance and dependence
Dependence involves μ receptors and is both physical and psychological in nature and is discussed in Chapter 5. If physical dependence develops, it is characterized by a definite withdrawal syndrome following cessation of drug treatment. This syndrome comprises a complex mixture of irritable, and sometimes aggressive, behaviour combined with extremely unpleasant autonomic symptoms such as fever, sweating, yawning and pupillary dilatation. The withdrawal syndrome is relieved by the administration of μ receptor agonists, and worsened by the administration of μ receptor antagonists.
Therapeutic notes
Weak opioid analgesics include pentazocine, codeine, dihydrocodeine and dextropropoxyphene: