OBJECTIVES
After studying this chapter, you should be able to:
Understand the functional significance of the gastrointestinal system, and in particular, its roles in nutrient assimilation, excretion, and immunity.
Describe the structure of the gastrointestinal tract, the glands that drain into it, and its subdivision into functional segments.
List the major gastrointestinal secretions, their components, and the stimuli that regulate their production.
Describe water balance in the gastrointestinal tract and explain how the level of luminal fluidity is adjusted to allow for digestion and absorption.
Identify the major hormones, other peptides, and key neurotransmitters of the gastrointestinal system.
Describe the special features of the enteric nervous system and the splanchnic circulation.
INTRODUCTION
The primary function of the gastrointestinal tract is to serve as a portal whereby nutrients and water can be absorbed into the body. In fulfilling this function, the meal is mixed with a variety of secretions that arise from both the gastrointestinal tract itself and organs that drain into it, such as the pancreas, gallbladder, and salivary glands. Likewise, the intestine displays a variety of motility patterns that serve to mix the meal with digestive secretions and move it along the length of the gastrointestinal tract. Ultimately, residues of the meal that cannot be absorbed, along with cellular debris, are expelled from the body. All of these functions are tightly regulated in concert with the ingestion of meals. Thus, the gastrointestinal system has evolved a large number of regulatory mechanisms that act both locally and over long distances to coordinate the function of the gut and the organs that drain into it.
STRUCTURAL CONSIDERATIONS
The parts of the gastrointestinal tract that are encountered by the meal or its residues include, in order, the mouth, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, and anus. Throughout the length of the intestine, glandular structures deliver secretions into the lumen, particularly in the stomach and mouth. Also important in the process of digestion are secretions from the pancreas and the biliary system of the liver. The intestine itself also has a very substantial surface area, which is important for its absorptive function. The intestinal tract is functionally divided into segments by means of muscle rings known as sphincters, which restrict the flow of intestinal contents to optimize digestion and absorption. These sphincters include the upper and lower esophageal sphincters, the pylorus that retards emptying of the stomach, the ileocecal valve that retains colonic contents (including large numbers of bacteria) in the large intestine, and the inner and outer anal sphincters. After toilet training, the latter permits delaying the elimination of wastes until a time when it is socially convenient.
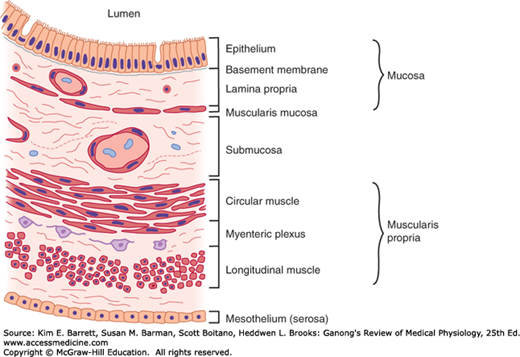
The intestine is composed of functional layers (Figure 25–1). Immediately adjacent to nutrients in the lumen is a single layer of columnar epithelial cells. This represents the barrier that nutrients must traverse to enter the body. Below the epithelium is a layer of loose connective tissue known as the lamina propria, which in turn is surrounded by concentric layers of smooth muscle, oriented circumferentially and then longitudinally to the axis of the gut (the circular and longitudinal muscle layers, respectively). The intestine is also amply supplied with blood vessels, nerve endings, and lymphatics, which are all important in its function.
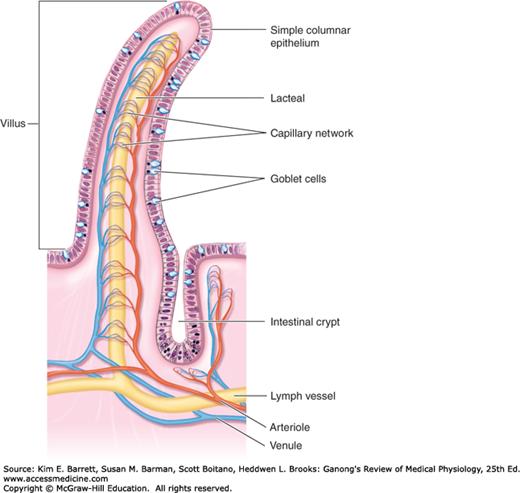
The epithelium of the intestine is also further specialized in a way that maximizes the surface area available for nutrient absorption. Throughout the small intestine, it is folded up into fingerlike projections called villi (Figure 25–2). Between the villi are infoldings known as crypts. Stem cells that give rise to both crypt and villus epithelial cells reside toward the base of the crypts and are responsible for completely renewing the epithelium every few days or so. Indeed, the gastrointestinal epithelium is one of the most rapidly dividing tissues in the body. Daughter cells undergo several rounds of cell division in the crypts then migrate out onto the villi, where they are eventually shed and lost in the stool. The villus epithelial cells are also notable for the extensive microvilli that characterize their apical membranes. These microvilli are endowed with a dense glycocalyx (the brush border) that probably protects the cells to some extent from the effects of digestive enzymes. Some digestive enzymes are also actually part of the brush border, being membrane-bound proteins. These so-called “brush border hydrolases” perform the final steps of digestion for specific nutrients.
FIGURE 25–2
The structure of intestinal villi and crypts. The epithelial layer also contains scattered endocrine cells and intraepithelial lymphocytes. The crypt base contains Paneth cells, which secrete antimicrobial peptides, as well as the stem cells that provide for continual turnover of the crypt and villus epithelium. The epithelium turns over every 3–5 days in healthy adult humans. (Reproduced with permission from Fox SI: Human Physiology, 10th ed. New York, NY: McGraw-Hill; 2008.)
GASTROINTESTINAL SECRETIONS
The first secretion encountered when food is ingested is saliva. Saliva is produced by three pairs of salivary glands (the parotid, submandibular, and sublingual glands) that drain into the oral cavity. It has a number of organic constituents that serve to initiate digestion (particularly of starch, mediated by amylase) and which also protect the oral cavity from bacteria (such as immunoglobulin A and lysozyme). Saliva also serves to lubricate the food bolus (aided by mucins). Secretions of the three glands differ in their relative proportion of proteinaceous and mucinous components, which results from the relative number of serous and mucous salivary acinar cells, respectively. Saliva is also hypotonic compared with plasma and alkaline; the latter feature is important to neutralize any gastric secretions that reflux into the esophagus.
The salivary glands consist of blind end pieces (acini) that produce the primary secretion containing the organic constituents dissolved in a fluid that is essentially identical in its composition to plasma. The salivary glands are actually extremely active when maximally stimulated, secreting their own weight in saliva every minute. To accomplish this, they are richly endowed with surrounding blood vessels that dilate when salivary secretion is initiated. The composition of the saliva is then modified as it flows from the acini out into ducts that eventually coalesce and deliver the saliva into the mouth. Na+ and Cl− are extracted and K+ and bicarbonate are added. Because the ducts are relatively impermeable to water, the loss of NaCl renders the saliva hypotonic, particularly at low secretion rates. As the rate of secretion increases, there is less time for NaCl to be extracted and the tonicity of the saliva rises, but it always stays somewhat hypotonic with respect to plasma. Overall, the three pairs of salivary glands that drain into the mouth supply 1000–1500 mL of saliva per day.
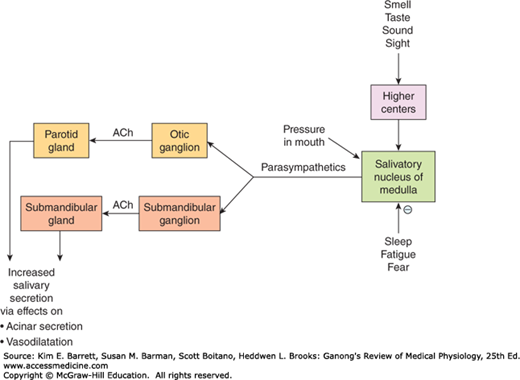
Salivary secretion is almost entirely controlled by neural influences, with the parasympathetic branch of the autonomic nervous system playing the most prominent role (Figure 25–3). Sympathetic input slightly modifies the composition of saliva (particularly by increasing proteinaceous content), but has little influence on volume. Secretion is triggered by reflexes that are stimulated by the physical act of chewing, but is actually initiated even before the meal is taken into the mouth as a result of central triggers that are prompted by thinking about, seeing, or smelling food. Indeed, salivary secretion can readily be conditioned, as in the classic experiments of Pavlov where dogs were conditioned to salivate in response to a ringing bell by associating this stimulus with a meal. Salivary secretion is also prompted by nausea but inhibited by fear or during sleep.
FIGURE 25–3
Regulation of salivary secretion by the parasympathetic nervous system. ACh, acetylcholine. Saliva is also produced by the sublingual glands (not depicted), but these are the minor contributors to both resting and stimulated salivary flows. (Adapted with permission from Barrett KE: Gastrointestinal Physiology. New York, NY: McGraw-Hill; 2006.)
Saliva performs a number of important functions: it facilitates swallowing, keeps the mouth moist, serves as a solvent for the molecules that stimulate the taste buds, aids speech by facilitating movements of the lips and tongue, and keeps the mouth and teeth clean. The saliva also has some antibacterial action, and patients with deficient salivation (xerostomia) have a higher than normal incidence of dental caries. The buffers in saliva help maintain the oral pH at about 7.0.
Food is stored in the stomach; mixed with acid, mucus, and pepsin; and released at a controlled, steady rate into the duodenum (Clinical Box 25–1).
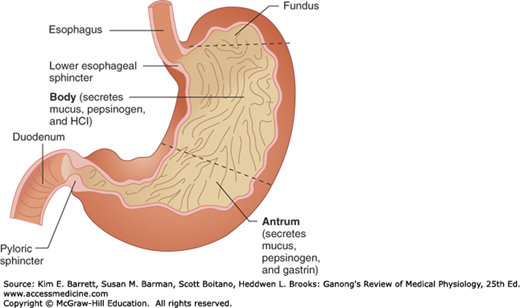
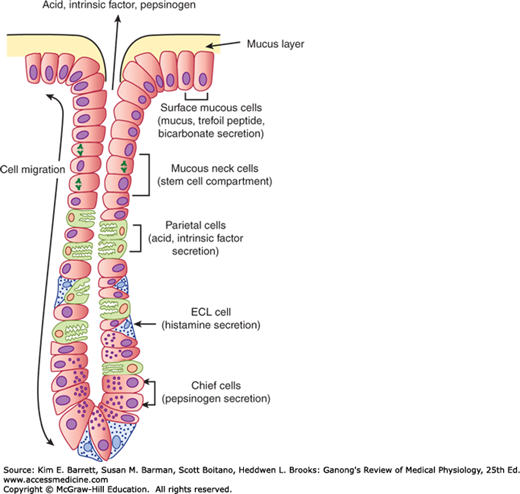
The gross anatomy of the stomach is shown in Figure 25–4. The gastric mucosa contains many deep glands. In the cardia and the pyloric region, the glands secrete mucus. In the body of the stomach, including the fundus, the glands also contain parietal (oxyntic) cells, which secrete hydrochloric acid and intrinsic factor, and chief (zymogen, peptic) cells, which secrete pepsinogens (Figure 25–5). These secretions mix with mucus secreted by the cells in the necks of the glands. Several of the glands open onto a common chamber (gastric pit) that opens in turn onto the surface of the mucosa. Mucus is also secreted along with HCO3− by mucus cells on the surface of the epithelium between glands.
FIGURE 25–5
Structure of a gastric gland from the fundus or body of the stomach. These acid- and pepsinogen-producing glands are referred to as “oxyntic” glands in some sources. Similarly, some sources refer to parietal cells as oxyntic cells. ECL, enterochromaffin-like. (Adapted with permission from Barrett KE: Gastrointestinal Physiology. New York, NY: McGraw-Hill; 2006.)
The stomach has a very rich blood and lymphatic supply. Its parasympathetic nerve supply comes from the vagi and its sympathetic supply from the celiac plexus.
The stomach also adds a significant volume of digestive juices to the meal. Like salivary secretion, the stomach readies itself to receive the meal before it is actually taken in, during the so-called cephalic phase that can be influenced by food preferences. Subsequently, there is a gastric phase of secretion that is quantitatively the most significant, and finally an intestinal phase once the meal has left the stomach. Each phase is closely regulated by both local and distant triggers.
The gastric secretions (Table 25–1) arise from glands in the wall of the stomach that drain into its lumen, and also from the surface cells that secrete primarily mucus and bicarbonate to protect the stomach from digesting itself, as well as substances known as trefoil peptides that stabilize the mucus-bicarbonate layer. The glandular secretions of the stomach differ in different regions of the organ. The most characteristic secretions derive from the glands in the fundus or body of the stomach. These contain the distinctive parietal cells, which secrete hydrochloric acid and intrinsic factor; and chief cells, which produce pepsinogens and gastric lipase (Figure 25–5). The acid secreted by parietal cells serves to sterilize the meal and also to begin the hydrolysis of dietary macromolecules. Intrinsic factor is important for the later absorption of vitamin B12, or cobalamin. Pepsinogen is the precursor of pepsin, which initiates protein digestion. Lipase similarly begins the digestion of dietary fats.
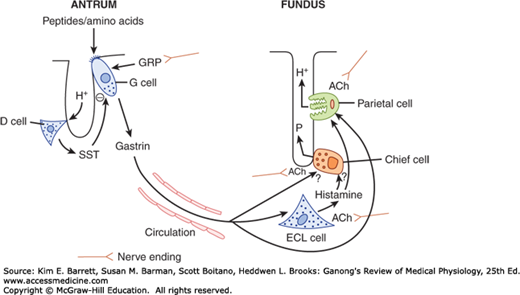
There are three primary stimuli of gastric secretion, each with a specific role to play in matching the rate of secretion to functional requirements (Figure 25–6). Gastrin is a hormone that is released by G cells in the antrum of the stomach both in response to a specific neurotransmitter released from enteric nerve endings, known as gastrin-releasing peptide (GRP) or bombesin, and also in response to the presence of oligopeptides in the gastric lumen. Gastrin is then carried through the bloodstream to the fundic glands, where it binds to receptors not only on parietal (and likely, chief cells) to activate secretion, but also on so-called enterochromaffin-like cells (ECL cells) that are located in the gland, and release histamine. Histamine is also a trigger of parietal cell secretion, via binding to H2-receptors. Finally, parietal and chief cells can also be stimulated by acetylcholine, released from enteric nerve endings in the fundus.
FIGURE 25–6
Regulation of gastric acid and pepsin secretion by soluble mediators and neural input. Gastrin is released from G cells in the antrum in response to gastrin releasing peptide (GRP) and travels through the circulation to influence the activity of enterochromaffin-like (ECL) cells and parietal cells. ECL cells release histamine, which also acts on parietal cells. Acetylcholine (ACh), released from nerves, is an agonist for ECL cells, chief cells, and parietal cells. Other specific agonists of the chief cell are not well understood. Gastrin release is negatively regulated by luminal acidity via the release of somatostatin from antral D cells. P, pepsinogen. (Adapted with permission from Barrett KE: Gastrointestinal Physiology. New York, NY: McGraw-Hill; 2006.)
CLINICAL BOX 25–1 Peptic Ulcer Disease
Gastric and duodenal ulceration in humans is related primarily to a breakdown of the barrier that normally prevents irritation and autodigestion of the mucosa by the gastric secretions. Infection with the bacterium Helicobacter pylori disrupts this barrier, as do aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit the production of prostaglandins and consequently decrease mucus and HCO3− secretion. The NSAIDs are widely used to combat pain and treat arthritis. An additional cause of ulceration is prolonged excess secretion of acid. An example of this is the ulcers that occur in the Zollinger–Ellison syndrome. This syndrome is seen in patients with gastrinomas. These tumors can occur in the stomach and duodenum, but most of them are found in the pancreas. The gastrin causes prolonged hypersecretion of acid, and severe ulcers are produced.
THERAPEUTIC HIGHLIGHTSGastric and duodenal ulcers can be given a chance to heal by inhibition of acid secretion with drugs such as omeprazole and related drugs that inhibit H+–K+ ATPase (“proton pump inhibitors”). If present, H. pylori can be eradicated with antibiotics, and NSAID-induced ulcers can be treated by stopping the NSAID or, when this is not advisable, by treatment with the prostaglandin agonist misoprostol. Gastrinomas can sometimes be removed surgically.
Gastric secretion that occurs during the cephalic phase is defined as being activated predominantly by vagal input that originates from the brain region known as the dorsal vagal complex, which coordinates input from higher centers. Vagal outflow to the stomach then releases GRP and acetylcholine, thereby initiating secretory function. However, before the meal enters the stomach, there are few additional triggers and thus the amount of secretion is limited. Once the meal is swallowed, on the other hand, meal constituents trigger substantial release of gastrin and the physical presence of the meal also distends the stomach and activates stretch receptors, which provoke a “vago-vagal” as well as local reflexes that further amplify secretion during the gastric phase. The presence of the meal also buffers gastric acidity that would otherwise serve as a feedback inhibitory signal to shut off secretion secondary to the release of somatostatin, which inhibits both G and ECL cells as well as secretion by parietal cells themselves (Figure 25–6). This probably represents a key mechanism whereby gastric secretion is terminated after the meal moves from the stomach into the small intestine.
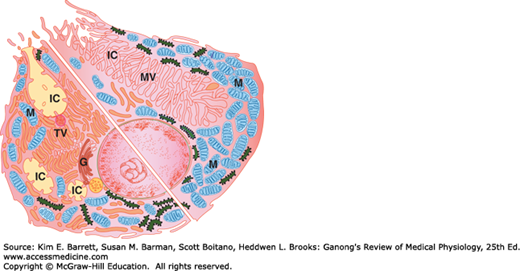
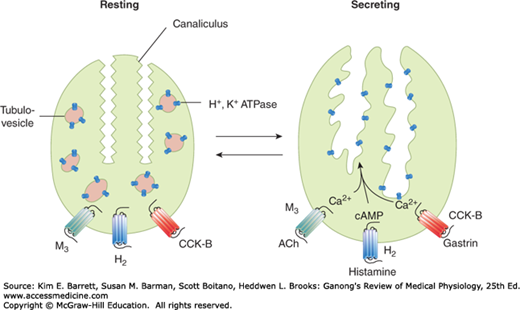
Gastric parietal cells are highly specialized for their unusual task of secreting concentrated acid (Figure 25–7). The cells are packed with mitochondria that supply energy to drive the apical H+, K+-ATPase, or proton pump, that moves H+ ions out of the parietal cell against a concentration gradient of more than a million-fold. At rest, the proton pumps are sequestered within the parietal cell in a series of membrane compartments known as tubulovesicles. When the parietal cell begins to secrete, on the other hand, these vesicles fuse with invaginations of the apical membrane known as canaliculi, thereby substantially amplifying the apical membrane area and positioning the proton pumps to begin acid secretion (Figure 25–8). The apical membrane also contains potassium channels, which supply the K+ ions to be exchanged for H+, and Cl− channels that supply the counterion for HCl secretion (Figure 25–9). The secretion of protons is also accompanied by the release of equivalent numbers of bicarbonate ions into the bloodstream, which are later used to neutralize gastric acidity once its function is complete (Figure 25–9).
FIGURE 25–7
Composite diagram of a parietal cell, showing the resting state (lower left) and the active state (upper right). The resting cell has intracellular canaliculi (IC), which open on the apical membrane of the cell, and many tubulovesicular structures (TV) in the cytoplasm. When the cell is activated, the TVs fuse with the cell membrane and microvilli (MV) project into the canaliculi, so the area of cell membrane in contact with gastric lumen is greatly increased. M, mitochondrion; G, Golgi apparatus. (Reproduced with pemission of Ito S, Schofield GC: Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol 1974; Nov; 63(2 Pt 1):364–382.)
FIGURE 25–8
Parietal cell receptors and schematic representation of the morphologic changes depicted in Figure 25–7. Amplification of the apical surface area is accompanied by an increased density of H+, K+–ATPase molecules at this site. Note that acetylcholine (ACh) and gastrin signal via calcium, whereas histamine signals via cAMP. (Adapted with permission from Barrett KE: Gastrointestinal Physiology. New York, NY: McGraw-Hill; 2006.)
The three agonists of the parietal cell—gastrin, histamine, and acetylcholine—each bind to distinct receptors on the basolateral membrane (Figure 25–8). Gastrin and acetylcholine promote secretion by elevating cytosolic free calcium concentrations, whereas histamine increases intracellular cyclic adenosine 3′,5′-monophosphate (cAMP). The net effects of these second messengers are the transport and morphologic changes described above. However, it is important to be aware that the two distinct pathways for activation are synergistic, with a greater than additive effect on secretion rates when histamine plus gastrin or acetylcholine, or all three, are present simultaneously. The physiologic significance of this synergism is that high rates of secretion can be stimulated with relatively small changes in availability of each of the stimuli. Synergism is also therapeutically significant because secretion can be markedly inhibited by blocking the action of only one of the triggers (most commonly that of histamine, via H2-antagonists that are widely used therapies for adverse effects of excessive gastric secretion, such as reflux).
Gastric secretion adds about 2.5 L/day to the intestinal contents. However, despite their substantial volume and fine control, gastric secretions are dispensable for the full digestion and absorption of a meal, with the exception of cobalamin absorption. This illustrates an important facet of gastrointestinal physiology, namely that digestive and absorptive capacities are markedly in excess of normal requirements. On the other hand, if gastric secretion is chronically reduced, individuals may display increased susceptibility to infections acquired via the oral route.
The pancreatic juice contains enzymes that are of major importance in digestion (see Table 25–2). Its secretion is controlled in part by a reflex mechanism and in part by the gastrointestinal hormones secretin and cholecystokinin (CCK).
| Source | Enzyme | Activator | Substrate | Catalytic Function or Products |
|---|---|---|---|---|
| Salivary glands | Salivary α-amylase | Cl− | Starch | Hydrolyzes 1:4α linkages, producing α-limit dextrins, maltotriose, and maltose |
| Stomach | Pepsins (pepsinogens) | HCl | Proteins and polypeptides | Cleave peptide bonds adjacent to aromatic amino acids |
| Gastric lipase | Triglycerides | Fatty acids and glycerol | ||
| Exocrine pancreas | Trypsin (trypsinogen) | Enteropeptidase | Proteins and polypeptides | Cleave peptide bonds on carboxylside of basic amino acids (arginineor lysine) |
| Chymotrypsins (chymotrypsinogens) | Trypsin | Proteins and polypeptides | Cleave peptide bonds on carboxyl side of aromatic amino acids | |
| Elastase (proelastase) | Trypsin | Elastin, some other proteins | Cleaves bonds on carboxyl side of aliphatic amino acids | |
| Carboxypeptidase A (procarboxypeptidase A) | Trypsin | Proteins and polypeptides | Cleave carboxyl terminal aminoacids that have aromatic or branched aliphatic side chains | |
| Carboxypeptidase B (procarboxypeptidase B) | Trypsin | Proteins and polypeptides | Cleave carboxyl terminal amino acids that have basic side chains | |
| Colipase (procolipase) | Trypsin | Fat droplets | Binds pancreatic lipase to oil droplet in the presence of bile acids | |
Pancreatic lipase Cholesteryl ester hydrolase | … … | Triglycerides Cholesteryl esters | Monoglycerides and fatty acids Cholesterol | |
Pancreatic α-amylase Ribonuclease | Cl− … | Starch RNA | Same as salivary α-amylase Nucleotides | |
Deoxyribonuclease Phospholipase A2 (pro-phospholipase A2) | … Trypsin | DNA Phospholipids | Nucleotides Fatty acids, lysophospholipids | |
| Intestinal mucosa | Enteropeptidase | … | Trypsinogen | Trypsin |
| Aminopeptidases | … | Polypeptides | Cleave amino terminal amino acid from peptide | |
| Carboxypeptidases | … | Polypeptides | Cleave carboxyl terminal amino acid from peptide | |
| Endopeptidases | … | Polypeptides | Cleave between residues in midportion of peptide | |
Dipeptidases Maltase Lactase | … … … | Dipeptides Maltose, maltotriose Lactose | Two amino acids Glucose Galactose and glucose | |
| Sucraseb | … | Sucrose; also maltotriose and maltose | Fructose and glucose | |
| Isomaltaseb | … | α-Limit dextrins, maltose Maltotriose | Glucose | |
| Nuclease and related enzymes | … | Nucleic acids | Pentoses and purine and pyrimidine bases | |
| Cytoplasm of mucosal cells | Various peptidases | … | Di-, tri-, and tetrapeptides | Amino acids |