Chapter 29 An overview of drugs with antihepatotoxic and oral hypoglycaemic activities
PLANTS IN THE TREATMENT OF LIVER AND BILIARY TRACT DISEASES
In order to reduce the number of animal experiments involved in these surveys H. Hikino and colleagues developed (1983–85) assay methods for antihepatotoxic activity using hepatotoxin-induced cytotoxicity in primary cultured hepatocytes. The fractionated plant extract under test and the hepatotoxin (e.g. CCl4) are added to the hepatocyte medium and incubated; the activity of the transaminases released intothe medium are then determined. The results obtained by this method were comparable with the in vivo assays. (For further details and references readers should consult H. Hikino in Biologically Active Natural Products, eds K. Hostettman; P. J. Lea, Proc. Phytochemical Soc. Europe, 1987, No. 27, p. 143; R. Gebhardt, Planta Med., 2000, 66, 99).
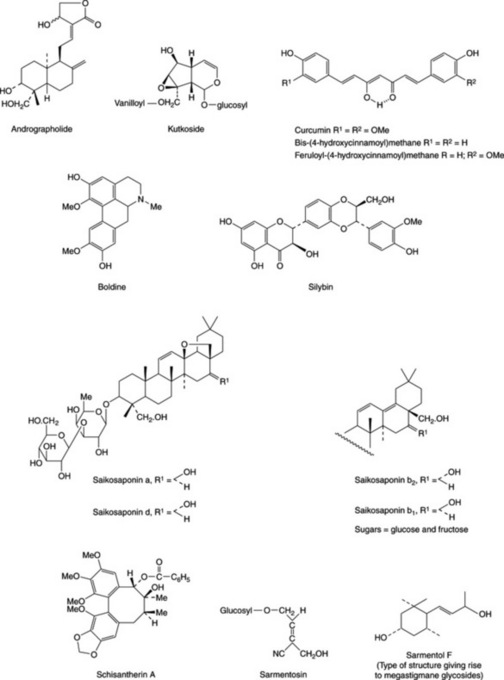
Handa and his group (Fitoterapia, 1991, 62, 229) reported that about 170 phytoconstituents isolated from 110 plants belonging to 55 families were stated to possess liver-protective activity; about 600 commercial herbal formulations with claimed hepatoprotective activity are being marketed world-wide. The active constituents elucidated to date involve a wide range of components including terpenoids, curcuminoids, lignoids, flavonoids, cyanogenetic glycosides etc., and some examples are given in Fig. 29.1. The terminal events in the attack on the liver by carbon tetrachloride involve the production of a highly reactive radical leading to lipid oxidation and the inhibition of the calcium pump of the microsome giving rise to liver lesions. Glycyrrhizin, glycyrrhetinic acid (Table 23.5) and wuweizisu C (Table 21.7) and gomisin A (lignoid constituents of Schizandra chinensis fruits) exert their activity as antioxidants. Similarly, the patented flavonoid extract (Kolaviron) of Garcinia kola seeds for the treatment of hepatic disorder has been shown to have antioxidant and scavenging properties E. O. Farombie et al., Pharm. Biol., 2002, 40, 107. Based on lignan content, high pressure liquid chromatography has proved useful for the identification and differentiation of samples of Schisandra chinensis and S. sphenanthera collected from different regions of China (Zhu Min et al., Chromatographia, 2007, 66, 125).
Milk-thistle fruit
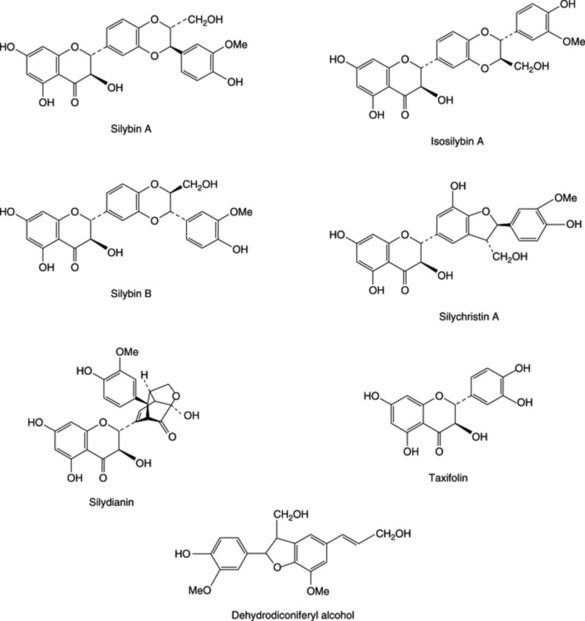
BP/EP, BHP 1996 consists of the mature fruit, devoid of pappus, of Silybum marianum L. Gaertner. Several so-called flavanolignans with marked hepatoprotective properties have been isolated from the fruits. A mixture of these, termed silymarin, is available commercially as a dried, purified and standarized extract. Principal components of the extract are three pairs of diastereoisomers: silybin (silibinin) A and silybin (silibinin) B, isosilybin (isosilibinin) A and isosilybin (isosilibinin) B and, silychristin (silicristin) and silychristin (silicristin) B; see Fig. 29.2 for formulae. Other related constituents are silandrin, 3-deoxysilychristin, silymonin and silydianin. A new compound, silyamandin, has recently been isolated from incubated tincture of milk thistle; it may arise from the degradation of silydianin (Fig. 29.2) (S. L. Mackinnon et al., Planta Medica, 2007, 73, 1214). The literature nomenclature for these compounds can be confusing as recommended international proprietary names (in brackets above) may also be used and both nomenclatures still appear to be used by authors. For isolation and structural reports on the above see DY-W. Lee and Y. Liu, J. Nat. Prod., 2003, 66, 1171, 1632; W. A. Smith et al., Planta Med., 2005, 71, 877. The levels of these flavonolignans vary markedly in samples of S. marianum from different sources; they are formed by various couplings of the flavonoid taxifolin and the lignan precursor coniferyl alcohol. Silymarin, in addition to the above flavonolignans, contains 20–30% of polyphenolic compounds. The scavenging (antioxidant) properties of the silymarin components have been tested against phenylglyoxylic ketyl radicals (F. Sersen et al., Fitoterapia, 2006, 77, 525).
Apart from the established use of silymarin as an antihepatotoxic agent, recent research together with clinical studies, have shown the flavonolignans to have anticancer properties (see Chapter 27).
Cynaria scolymus (Asteraceae/Compositae)
The leaves of the globe artichoke have been described in Chapter 19, the drug being used principally as a cholagogue (stimulation of bile production in the liver and promotion of emptying of the gall bladder and bile ducts). There has been more recent interest in the hepatoprotective properties of the plant and significant antioxidative activity has been demonstrated involving chlorogenic acid and cynarin; other constituents are, however, also implicated. For further details and references, see J. Barnes et al., Herbal Medicines, 3rd ed., 2007, p. 67. Pharmaceutical Press, London.
Peumus boldus
Boldo leaves (BP/EP; BHP 1996) are collected from the small tree Peumus boldus Molina (Monimiaceae) indigenous to Chile. The leaves are up to about 8 cm long, of coriaceous texture with a strong camphoraceous lemony odour owing to the presence of volatile oil (2%) containing ascaridole, linalool, p-cymene and cineole. E. Miraldi et al. (Fitoterapia, 1996, 67, 227) identified 46 components of the oil, 22 of which were recorded for the first time in P. boldus. The active constituents have been shown by laboratory testing to be alkaloids of the aporphine type (1–3%) the chief of which is boldine (Fig. 29.1).Boldine is reported to be a potent scavenger of hydroxyl, lipid and alkyl peroxyl radicals and experimental evidence supports its cytoprotective and anti-inflammatory properties (M. Gotteland et al., Planta Medica, 1997, 63, 311).
Taraxacum officinale root (Compositae). Syn: Dandelion root
The drug consists of the dried vertical rhizome and tap-root which pass imperceptibly into one another. Up to 30 cm long when fresh and2.5 cm diameter, it is frequently branched with an apical crown bearing brownish hairs. The rhizome has a ring of vascular bundles and a pith; the root has a central yellow wood. Concentric zones of latex vessels occur in the thick bark. Dandelion root contains up to 25% of inulin and other polysaccharides, sesquiterpene lactones including taraxacoside, triterpenes including taraxerol, taraxol and B-amyrin, various acids including caffeic and p-hydroxyphenylacetic acids, and carotenoid yellow colouring matter.
In addition to its use in medicine the dandelion root is useful botanically for the demonstration of latex vessels (Fig. 42.7) and inulin (Fig. 42.1). The leaf has similar medicinal uses. Flavonoids, cinnamic acids and coumarins from different plant tissues and medicinal preparations have been recorded (C. A. Williams et al., Phytochemistry, 1996, 42, 121). For a review of the phytochemistry and pharmacology of the drug (over 90 refs + formulae), see K. Schultz et al., J. Ethnopharmacol. 2006, 107, 313.
Hydrastis canadensis (Ranunculaceae)
The root is employed in atonic dyspepsia with hepatic symptoms. A fuller description of the drug is given in Chapter 26.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree