Fig. 32.1.
Ovarian serous surface inclusion lined by a flattened serous epithelium. Serous surface inclusions lack associated granulosa cells or luteinized stromal cells.
Follicular Cyst
Clinical
♦
Follicular cysts develop from preovulatory follicles and can be associated with pain, vomiting, diarrhea, constipation, and dysfunctional uterine bleeding. Most will regress within 2 months
♦
Occasionally, ovarian torsion may occur
Macroscopic
♦
By definiti on, these cysts are greater than 3 cm in diameter, but they may reach 10 cm or more. They have a smooth lining
Microscopic
♦
Follicle cysts are lined by granulosa cells, but the lining may be inapparent or absent in some areas. The surrounding theca cells may be luteinized
Corpus Luteum Cyst
Clinical
♦
Corpus luteum cysts develo p from postovulatory follicles (corpora lutea) during menstruation or pregnancy. They are usually asymptomatic
♦
Similar to follicular c ysts and spontaneous regression, it is typical
Macroscopic
♦
These cysts are usually 2–3 cm in diameter and have a central hemorrhage surrounded by a lining of fibrous connective tiss ue that in turn is surrounded by a convoluted yellow layer of tissue
Microscopic
♦
The yellow layer is c omposed of luteinized granulosa cells with abundant eosinophilic cytoplasm
Large Solitary Luteinized Follicle Cyst of Pregnancy and Puerperium
♦
This is a variant of follicular cyst that may present as a palpable adnexal mass or may be seen during a cesarean section. It may be up to 25 cm in diameter, and the wall is compose d of luteinized granulosa and theca cells (Fig. 32.2). Prominent nuclear atypia may be seen, but mitotic figures are absent and behavior is benign
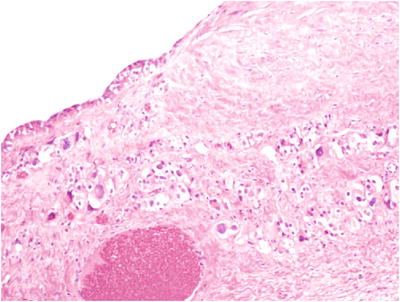

Fig. 32.2.
Large solitary luteinized follicle cyst of pregnancy and puerperium. The cyst is lined by granulosa cells and luteinized stromal cells are present in the fibrous tissue of the cyst wall.
Hyperreactio Luteinalis (Multiple Luteinized Follicular Cysts)
Clinical
♦
Hyperreactio luteinalis is a bilateral ovarian enlargement due to multiple luteinized follicular cysts resulting from hCG stimulation. The hCG elevation may result from a molar gestation, a multiple gestation, or a hydropic fetus. Hyperreactio luteinalis occurs in up to 25% of cases of gestational trophoblastic disease
♦
This lesion regresses following the removal of the hCG stimulus
Macroscopic
♦
The ovaries are enlarged (up to 15 cm) and are composed of multiple smooth-walled cysts
Microscopic
♦
Microscopically, the cysts are lined by granulosa cells surrounded by theca cells, both of which may be luteinized
Differential Diagnosis
♦
The ovaries may grossly resemble the ovaries of polycystic ovary syndrome, but the clinical situation differs (while hyperreactio luteinalis is usually associated with an abnormal gestation, polycystic ovary syndrome is commonly associated with infertility). Also, the ovaries lack the thick fibrous capsule and smooth surface of polycystic ovary syndrome
Luteoma of Pregnancy (Pregnancy Luteoma)
Clinical
♦
These no dular hyperplastic masses are found most commonly incidentally during cesarean section or postpartum tubal ligation
♦
About one-quarter of patients will have virilization or hirsutism
♦
Incompletely resected tumors regress following the pregnancy
Macroscopic
♦
Grossly, they are composed of red-brown soft tissue, and they may be multiple or bilateral. They are commonly 6–12 cm in diameter. Necrosis may be seen postpartum
Microscopic
♦
Microscopically, they are composed of polygonal luteinized cells in nodules or of a diffuse pattern with abundant eosinophilic cytoplasm and round nuclei (Fig. 32.3). Small cysts may be present
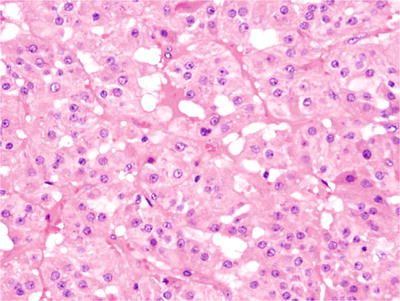

Fig. 32.3.
Luteoma of pregnancy. The tumor was an incidental finding during a cesarean section and was composed of polygonal luteinized cells with abundant eosinophilic cytoplasm.
Differential Diagnosis
♦
Some sex cord–stromal tu mors may resemble luteoma of pregnancy, but it is unusual for those to be bilateral, in contrast to the commonly bilateral luteoma of pregnancy. The tumors that may appear histologically similar include Leydig cell tum or, thecoma, sclerosing stromal tumor, and juvenile granulosa cell tumors
Polycystic Ovary Syndrome (Stein–Leventhal Syndrome)
Clinical
♦
Polycystic ovary syndrome is a rel atively common (5–10% of the female population) disorder characterized by chronic anovulation, multicystic ovaries with a fibrotic cortex, and hyperandrogenism. Clinical findings include dysfunctional uterine bleeding, infertility, obesity, and hirsutism
♦
Many patients will have androgen excess, with an elevation of serum testosterone, dehydroepiandrosterone, or other androgens
♦
Some patients may also have abnormal glucose tolerance, insulin resistance, and acanthosis nigricans (“HAIR-AN syndrome”: hyperandrogenism, insulin resistance, and acanthosis nigricans)
Macroscopic
♦
The ovaries are grossly enlarged (approximately twice the normal size) with a smooth outer surface, a thick fibrous cortex, and multiple small (<1 cm) cysts just under the fibrotic cortex
Microscopic
♦
A thickened fibro us capsule at the surface of the ovary is seen with multiple follicle cysts underneath. The cysts are lined by a thinned granulosa cell layer with surrounding theca cells. Evidence of ovulation (such as corpora albi cantia or corpora lutea) is not seen
Differential Diagnosis
♦
See hyper reactio luteinalis abo ve
Stromal Hyperthecosis/Stromal Hyperplasia
Clinical
♦
These disorders occ ur in perimenopausal or postmenopausal women and may be associated with virilization, increased serum testosterone, hypertension, or decreased glucose tolerance and are seen as bilateral ovarian enlargement
Macroscopic
♦
Grossly, the ovaries are enlarged (up to twice normal size), firm, and white, tan, or yellow in color. In some cases, stromal hyperthecosis may be grossly nodular
Microscopic
♦
Microscopic ally, the ovaries have a hyperplastic stroma with luteinized stromal cells (stromal hyperthecosis) or densely arranged nonluteinized stromal cells (stromal hyperplasia)
Leydig Cell Hyperplasia (Hilus Cell Hyperplasia)
Clinical
♦
Small nests of Leydig cells can be found in t he ovarian hilus where they are known as hilus cells and they occasionally may be found elsewhere in the ovary or in the fallopian tube. Proliferation of hilus cells is observed in pregnancy, after menopause, and in several pathologic conditions including stromal hyperplasia and rete cysts. Leydig cell hyperplasia is usually not clinically significant, but can be associated with increased serum testosterone
Macroscopic
♦
Leydig cell hyperplasia is not grossly apparent
Microscopic
♦
Leydig cell hyperplasia is seen as nodules of polygonal Leydig cells with abundant eosinophilic granular cytoplasm and round nuclei. Reinke cry stals (eosinophilic rod-shaped cytoplasmic crystalloids) and cytoplasmic yellow-brown lipofuscin pigment may be seen
Differential Diagnosis
♦
Masses greater tha n 1 cm com posed of Leydig cells may be considered Leydig cell tumors
Rete Cyst
Clinical
♦
Cysts of th e rete ovarii may occur in the ovarian hilus
Macroscopic
♦
They are usually unilocular and small, but occasionally, cysts up to 12 cm can occur
Microscopic
♦
Microscopically, they are lined by flattened or cuboidal cells. Smooth muscle and nests of hyperpl astic Leydig cells may be seen adjacent to the cyst
Massive Ovarian Edema
Clinical
♦
Massive ov arian edema may clinically mimic a neoplasm and often presents with abdominal pain. Patients are young (usually in the second or third decade)
♦
The frequent association with torsion suggests that development of massive ovarian edema may be related to int ermittent or partial ovarian torsion that impairs lymphatic and venous drainage from the ovary
Macroscopic
♦
The ovary is grossl y greatly enlarged (over 10 cm and occasionally up to 35 cm) and white in color. The cut surface shows edematous-appearing fibrous tissue
Microscopic
♦
Microscopically, th e ovary is composed of cytologically bland stromal cells widely separated by edema fluid (Fig. 32.4)
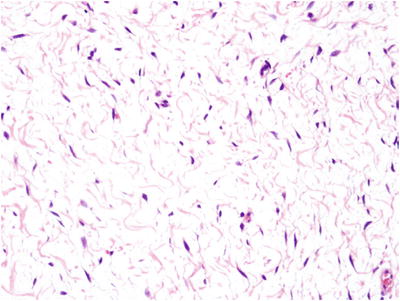

Fig. 32.4.
Massive ovarian edema. This massively enlarged ovary was grossly white in color with a fibrous and edematous cut surface. Microscopically, cytologically bland, widely spaced spindle cells were seen with occasional collagen fibers.
Differential Diagnosis
♦
Grossly, metastati c carcinomas with mucin production or edematous tumors (such as some lymphomas) may resemble massive ovarian edema. Sclerosing stromal tumors may have edematous areas that microscopically resemble massive ovarian edema
Ovarian Torsion
Clinical
♦
Ovarian torsion is freque ntly associated with a mass lesion, usually a benign cyst or tumor, but torsion of an otherwise normal ovary may also occur. It occurs most commonly in women of reproductive age, but about one-fourth of cases occur in children. Ovarian torsion in children is associated with a malignant tumor in less than 2% of patients. Patients present with abdo minal pain that may mimic appendicitis
Macroscopic
♦
The ovary is grossly swollen (up to 6 cm), hemorrhagic, and, sometimes, infarcted
Microscopic
♦
Hemorrhage, edema, and ischemic necrosis are seen. A search should be made for a possible associated neoplasm, but identification of one, even if present, may be difficult due to infarction
Ectopic Decidual Reaction
♦
Decidual reac tion may be seen in the ovarian stroma as a result of pregnancy or progestin treatment or adjacent to a corpus luteum. The stromal cells are in the form of small nodules or large sheets and have the typical abundant eosinophilic cytoplasm of endometrial decidual cells. Focally, nuclear atypia or cytoplasmic vacuolization (a potential mimic of signet-ring adenocarcinoma, but PAS negative) may be seen
Endometriosis
Clinical
♦
Endometriosis is defined as the presence of benign endometrial glands and stroma, often with evidence of hemorrhage outside the uterus. While the etiology of the condition is incompletely understood, most cases probably result from retrograde menstruation through the fallopian tube with implantation of endometrial tissue on the ovary or peritoneum. Other possibilities include metaplasia of the pelvic peritoneum or, rarely, lymphovascular dissemination of benign endometrial tissue
♦
Endometriosis affects 4–10% of women of reproductive age, and it is associated with dysmenorrhea and chronic pelvic pain. Up to 30% of women with endometriosis are infertile. Endometriosis is associated with a three- to eightfold increased incidence of ovarian carcinomas and borderline tumors
♦
Low parity and cervical stenosis are risk factors
♦
Serum CA-125 may be elevated in women with endometriosis. The ovary and any pelvic or abdominal serosal surface may be involved. Surgical scars (cesarean section scars, episiotomy sites, or scars from other abdomi nal surgeries) may be involved
♦
Rarely, carcinomas may arise within endometriosis in the colon or elsewhere
Macroscopic
♦
Grossly, small le sions may be seen as patches of blue, red, or brown on a serosal surface. Microscopically, endometrial glands, stroma, and hemosiderin-containing macrophages are seen
♦
The ovary is the most common location for endometriotic cysts. These cysts may present as a large mass lesion and may largely replace the normal ovarian tissue. The cysts contain brown degenerated blood (“chocolate cysts”)
♦
Other locations for endometriosis include the fallopian tube, uterine serosa, uterine cervix, colon, and the peritoneu m (especially in the cul-de-sac). Very rarely, the lung or pleura may be involved
Microscopic
♦
Noncystic lesions of endometriosis are seen as endometrioid glands, which commonly are inactive appearing but may hav e a proliferative or secretory appearance. The stroma may be densely cellular (resembling proliferative endometrial stroma) or edematous (resembling secretory phase stroma) or may show decidual change. Macrophages are commonly seen, but hemosiderin pigment may or may not be visible
♦
Endometriotic cysts are lined by endometrial epithelium with underlying endometrial stroma, hemorrhage, and pigmented (hemosiderin-containing) macrophages
♦
Decidual reaction, Arias-Stella reaction, hyperplasia, and cytologic atypia may be seen in endometriosis. Malignant tumors also may arise within endometriosis. Clear cell adenocarcinomas are the most common endometriosis-associated carcinoma, but endometrioid adenocarcinomas may also be seen
♦
Occasional cases of endometriosis, usually in the ovary, may have a paucity or absence of glands (“benign stromatosis”)
♦
Occasionally, the endometrial stroma in an endometriotic cyst may undergo smooth muscle m etaplasia (“endomyometriosis”). This phenomenon may result in the presence of a “uterus-like mass” in the ovary or elsewhere
Differential Diagnosis
♦
Grossly, corpus luteum cysts appear as a hemorrhagic cystic lesion in the ovary similar to endometriosis but have a surround ing rim of yellow tissue
♦
The epithelium of endometriosis may be focally ciliated, but uniformly ciliated glands not associated with endometrial stroma are considered endosalpingiosis (see “Endosalpingiosis” in the peritoneum section below)
♦
Endometriotic glands may be irregular in outline but should not have extensive confluent growth. If this is seen, e ndometrioid carcinoma must be considered
Surface Epithelial Tumors
General Information concerning Surface Epithelial Tumors
♦
Ovarian c arcinomas comprise approximately 30% of all gynecologic tract malignancies. Surface epithelial tu mors (also known as surface epithelial-stromal tumors) are the most common type, comprising 90% of ovarian malignancies
♦
Risk factors for ovarian surface epithelial malignancies include postmenopausal estrogen replacement therapy and obesity. A history of oral contraceptive use and high parity (both associated with reduced ovulation) are associated with a decreased risk
♦
The mean 5-year survival for ovarian malignancies is only 32% as most patients present with advanced stage (outside the pelvis) disease and overall survival has not improved significantly in recent decades
♦
The most common genetic abnormality in familial cases of ovarian carcinoma is BRCA1 mutation. BRCA2 and hereditary non-polyposis colorectal carcinoma syndrome (Lynch syndrome) gene abnormalities (MLH1 and MSH2) are less important
♦
Immunophenotyping is useful in the differential diagnosis (Table 32.1)
Table 32.1.
Immunohistochemical Findings in Ovarian Tumors
CK7 | CK20 | PAX8 | WT1 | CA125 | P53 | CDX2 | TTF1 | Inh | Cal | Ch | OCT | PLAP | AFP | LCA | CD30 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Serous (HG) | + | u− | + | + | u+ | u+ | − | v | − | u− | − | − | v | − | nd | nd |
Mucinous | + | v | u+ | − | u− | u− | v | − | − | v | nd | − | v | nd | nd | nd |
Endometrioid | + | u− | + | u− | u− | v | u− | − | − | u− | nd | − | v | − | nd | nd |
Clear | + | − | + | − | − | − | v | − | − | u− | nd | u− | v | u− | nd | nd |
Brenner | + | − | nd | v | − | nd | nd | − | − | u− | nd | − | nd | nd | nd | nd |
Colorectal | v | v | nd | nd | u− | v | + | − | + | − | − | − | nd | − | nd | nd |
Appendiceal | v | + | nd | nd | nd | nd | + | − | nd | − | nd | nd | nd | nd | nd | nd |
Gastric | v | v | nd | nd | nd | v | v | − | − | − | − | − | nd | − | nd | nd |
Breast | + | − | nd | nd | u− | v | − | − | − | u− | nd | − | − | − | nd | nd |
Granulosa | nd | nd | v | v | − | − | − | nd | + | + | nd | − | − | − | nd | nd |
Sertoli–Leydig | − | nd | v | + | − | nd | nd | nd | + | + | nd | − | − | v | nd | nd |
Fibroma–thecoma | v | nd | − | + | − | nd | nd | nd | u+ | u+ | nd | − | − | − | nd | nd |
Dysgerminoma | nd | nd | nd | nd | − | − | u− | nd | − | − | − | u+ | + | − | − | − |
Yolk sac tumor | u− | nd | nd | − | nd | − | nd | nd | − | − | − | − | v | u+ | − | − |
Embryonal | u+ | − | nd | nd | nd | v | − | − | − | − | − | + | u+ | v | − | u+ |
Choriocarcinoma | nd | nd | nd | nd | nd | nd | nd | nd | + | nd | − | − | + | − | − | − |
Carcinoid | nd | nd | nd | nd | nd | nd | nd | v | − | − | + | − | − | nd | nd | − |
Lymphoma | nd | nd | nd | nd | nd | v | nd | nd | − | − | − | − | − | nd | + | v |
Mesothelioma | nd | − | nd | nd | v | v | − | − | − | u+ | v | − | − | − | − | u− |
DSRCT | nd | − | nd | nd | v | nd | nd | nd | nd | u− | nd | nd | nd | − | − | nd |
♦
Epithelial ovarian carcinomas have been divided into two broad categories, type I and type II tumors, based on clinic al, histopathologic, and molecular features
♦
The type I tumors include low-grade serous carcinoma, low-grade endometrioid carcinoma, mucinous carcinoma, and clear cell carcinoma, develop from precursors such as borderline tumors and endometriosis, are commonly confined to one ovary at presentation, are relatively indolent, and typically have mutations in genes such as KRAS, BRAF, PTEN, and ARID1A
♦
The type II tumors include high-grade serous carcinoma, high-grade endometrioid carcinoma, and malignant mixe d Müllerian tumor, develop from serous tubal intraepithelial neoplasia of the fallopian tube or from ovarian epithelium-lined inclusion cysts, typically present at advanced stage, are aggressive, have TP53 mutations in over 95% of cases, and are chromosomally unstable
♦
Recently, it has been suggested that the two-type classification is excessively simplistic and, based on differences in behavior, treatment response, and molecular pathology, that reduction to less than five categories (hig h-grade serous carcinoma, low-grade serous carcinoma, mucinous carcinoma, endometrioid carcinoma, and clear cell carcinoma) is inappropriate
High-Grade Serous Carcinoma
Clinical
♦
These tumors are aggressive and typically present at advanced stage and with ascites, with only ap proximately 1% of high-grade serous carcinomas confined to the ovary at diagnosis. Screening has not significantly changed this percentage
♦
Incidence of this tumor is declining, probably due to the protective effect of oral contraceptives
♦
Chemotherapy is effective in treating metastatic disease, with a response rate of about 80%, but the tumor consistently develops resistance to treatment and recurrence
Macroscopic
♦
Serous carcin oma is bilateral in two-thirds of cases
♦
Tumors vary from microscopic to over 20 cm. The cut surfaces of the tumor are commonly white to tan, and grossly apparent papillary structures, cysts, necrosis, and hemorrhage may be seen
Microscopic
♦
Neoplastic serous epithelium ranges from cytologically bland columnar epithelium resembling fallopian tube epithelium to solid nests of epithelium with markedly pleomorphic, enlarged, and hyperchromatic nuclei and scant cytoplasm
♦
Serous carcinoma was formerly known as “papillary serous carcinoma” and does commonly have papillary architecture, but tumors also may have entirely solid or glandular/cribriform pattern. “Transitional cell carcinoma of the ovary” has been described, but based on immunophenotype, molecular features, and coexistence with more typical high-grade serous carcinoma (or occasionally endometrioid carcinoma), this appears to be a pattern of high-grade serous carcinoma rather than a distinct tumor type
♦
Psammoma bodies are seen in about one-third of high-grade serous carcinomas, but they are also seen in low-grade serous carcinoma, serous tumors of low malignant potential, occasional nonovarian neoplasms, and benign mesothelial hyperplasia
♦
Serous carcinoma is usually positive for keratin 7, EMA, and CA125 and is negative for keratin 20 and cal retinin
♦
Mutations of TP53 are critical to the development of high-grade serous carcinoma and are seen in over 95% of these tumors. Two patterns of p53 immunohistochemical staining are seen in these tumors with this nuclear marker: strong diffuse staining (indicating a missense mutation) or complete absence of staining (in dicating a nonsense mutation with a truncated protein). The type I carcinomas, in contrast, typically have wild-type TP53 with patchy staining
♦
In the past, a three-grade system had been used for serous carcinoma. More recently, a two-grade system has been recommended. The two-grade system recognizes that low- and high-grade serous carcinomas are separate entities in terms of clinical behavior, precursor lesions, and molecular pathology. Furthermore, the prognosis does not differ between grade 2 and grade 3 tumors, using the three-grade system
♦
Morphologically, differentiation from low-grade serous carcinoma is made based on nuclear pleomorphism and mitotic rate. High-grade tumors have 3:1 nuclear size variation and more than 12 mitoses per 10 high power fields
♦
High-grade tumors (which make up approximately 90% of serous carcinomas) usually have TP53 mutations, lack KRAS mutations, and only very rarely arise from a serous tumor of low malignant potential or a low-grade serous carcinoma, while low-grade serous carcinomas (Fig. 32.6) commonly have KRA S mutations, lack TP53 mutations, and are more likely to arise from a serous tumor of low malignant potential
Molecular Pathology
♦
Serous carcinoma had been thought to arise from ovarian surface epithelium inclusions, but recent evidence supports the idea that many of these tumors originate from TP53-mutated precursor lesions within the fallopian tube . These lesions are referred to as serous tubal intraepithelial carcinoma (STIC). This was first suspected based on detection of intraepithelial carcinomas and small, clinically occult carcinomas in the fimbria of prophylactic salpingo-oophorectomy specimens from patients with BRCA1 and BRCA2 mutations
♦
Further support for fallopian tube origin of “ovarian” high-grade serous carcinoma includes gene expression studies (including TP53) that demonstrate similarity between “ovarian” high-grade serous carcinoma and STIC and the frequent identification of STIC in association with “ovarian” high-grade sero us carcinoma
Differential Diagnosis
♦
Endometrioid carcinoma, especially when poorly differentiated, may resemble serous carcinoma. Both may have papillary, glandular, or solid architecture or a mixture of these patterns. High-grade serous carcinoma s have higher grade nuclear atypia with occasional large, hyperchromatic nuclei, and the glands and papillary structures tend to have scalloped or irregular surfaces and slit-like lumens. Psammoma bodies are seen in about one-third of high-grade serous carcinoma cases. Endometrioid carcinoma glands tend to have round lumens with flat or continuous luminal surfaces and cells with a more columnar appearance. Squamous differentiation is seen in about one-third of endometrioid carcinoma cases, but may rarely also be seen in serous carcinoma
Low-Grade Serous Carcinoma
Clinical
♦
These tum ors are usually unilateral and may be confined to the ovary at diagnosis
♦
Low-grade serous carcinomas commonly arise in serous cystadenomatous tumors of low malignant potential (serous borderline tumors)
♦
Five-year survival is approximately 85% and 10-year survival is approximately 50%, but the tumor respond s poorly to platinum-based chemotherapy
Microscopic
♦
These tumors are co mposed of infiltrating small papillary epithelial cell groups, often surrounded by a retraction space, and the low power appearance is characteristic
♦
Differentiation from high-grade serous carcinoma is made based on nuclear pleomorphism and mitotic rate. Low-grade tumors lack 3:1 nuclear size variation and have less than 12 mitoses per 10 high power fields
♦
Psammoma bodies are typically abundant in low-grade serous carcinomas and in some cases there may be a gr eater volume of psammoma bodies than residual epithelium (the so-called psammocarcinoma, Fig. 32.5)
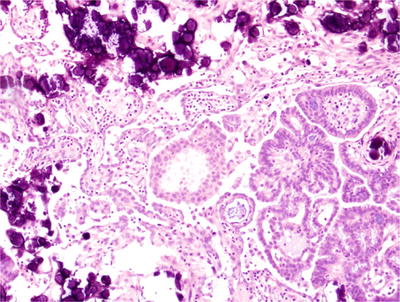

Fig. 32.5.
Low-grade serous carcinoma with abundant psammoma bodies (“psammocarcinoma”). This low-grade ovarian serous carcinoma has numerous laminated calcifications (psammoma bodies) that compose almost the same area on the slide as the malignant epithelium.
Serous Borderline Tumor (Serous Tumor of Low Malignant Potential, Atypical Proliferative Serous Tumor)
Clinical
♦
Borderline ovaria n tumors may be serous (approximately 2/3 of cases) or mucinous (approximately 1/3 of cases)
♦
Patients with serous tumors of low malignant potential are on average younger than patients with serous carcinoma (approximately 45 years old vs. 60 years for serous carcinoma). The tumors may be are bilateral and 22% of cases are associated with implants
♦
Prognosis is far better than serous carcinoma, with 99% 5-year survival for stage I cases and 55–75% 5-year survival for stage III. Mortality is seen in tumors that progress to low-grade s erous carcinoma
Macroscopic
♦
These tumors are commonly cystic with grossly identifiable nodularity or papillary structures in the cyst lining
Microscopic
♦
Serous tumors of low malignant potential (serous borderline tumors) have papillary architecture (with fibrovascular cores) and low-grade nuclear atypia (Figs. 32.6 and 32.7). Papillary tufting and epithe lial hyperplasia of several cells in thickness are present, but stromal invasion and extensive solid or cribriform architecture are absent
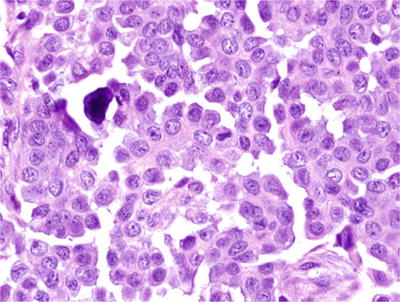
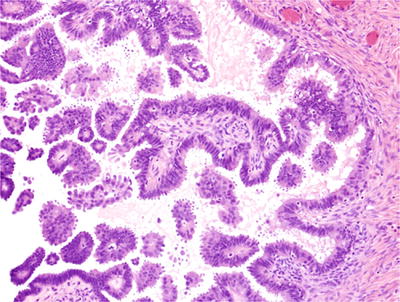

Fig. 32.6.
Low-grade serous carcinoma. The nuclei are enlarged but relatively uniform with only mild-to-moderate nuclear atypia and less than 12 mitoses per 10 high power fields.

Fig. 32.7.
Serous cystadenomatous tumor of low malignant potential (serous borderline tumor). This cystic tumor has papillary architecture with papillae covered by serous epithelium with mild nuclear atypia, nuclear stratification, and papillary tufting. Solid, cribriform, or invasive growth is not seen.
♦
A micropapillary variant of serous tumor of low malignant potential (comprising 5–15% of cases) exists. This tumor has thin micropapillae, without stromal support that are at least five times as long as they are wide arising directly from the surface of papillary structures with stromal cores (“nonhierarchical branching” or “medusa-head” appearance, Fig. 32.8) in at least a continuous 5 mm area. This variant (also termed, by some, “noninvasive low-grade serous carcinoma”) is associated with an increased likelihood of advanced stage and may have worse prognosis
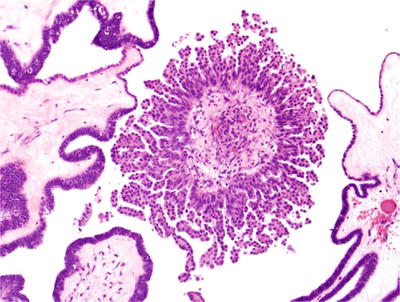

Fig. 32.8.
Micropapillary architecture. This serous tumor of low malignant potential had focal micropapillary architecture with thin micropapillae, with length greater than five times their width and lacking stromal cores.
♦
Peritoneal surface implants may be seen in association with serous tumors of low malignant potential. Noninvasive implants may be epithelial type (not associated with fibrotic stroma) or desmoplastic type. Noninvasive implants have minimal impact on prognosis, but invasive implants have behavior similar to low-gr ade serous carcinoma and it is now recommended that they should be diagnosed as foci of low-grade serous carcinoma
♦
Inclusions of serous epithelium with features of a low malignant potential tumor or endosalpingiosis may be seen in pelvic lymph nodes in association with ovarian serous tumors of low malignant potential. These may represent metastatic or independently arising tumors and do not appear to affect survival (if features sufficient for a diagnosis of low-grade serous carcinoma are absent)
Differential Diagnosis
♦
The usual considerations in the differential diagnosis are serous carcinoma (which has an invasive, cribriform/in terconnecting glandular, or solid pattern) and serous cystadenoma/serous cystadenofibroma (which may have papillary architecture within the cyst but lacks nuclear stratification and papillary tufting)
♦
Serous tumor of low malignant potential with microinvasion is diagnosed when clusters of tumor cells in the stroma measuring less than 5 mm are seen. This is seen in approximately 5% of serous tumors of low malignant potential and does not appear to alter progression-free survival
♦
Occasional metastatic tumors, such as pancreatic adenocarcinoma, may mimic serous low malignant potential tumors
Benign Serous Tumors
Macroscopic
♦
These lesions ar e greater than 1 cm; smaller lesions are considered cortical inclusion cysts
♦
Benign serous tumors (serous cystadenoma and serous cystadenofibroma) are cystic and are bilateral in approximately 20% of cases. Nodularity/papillary structures may be apparent in the cyst wall (this does not necessarily indicate that the tumor is a low malignant potential tumor)
Microscopic
♦
Serous cystadenomas are lined by a flat (one cell layer) epithelium that may be ciliated
♦
Serous cystadenofibromas have papillary architecture with the flat epithelium covering broad, “club-like” fibrous stromal cores. Some cases may also have a glandular pattern with dense fibrous stroma surrounding the glands
♦
Foci of epithelial changes, similar to those seen in a serous borderline tumor but involving less than 10% of the neoplasm, are termed “serous cystadenoma with focal epithelial proliferation” and benign behavior is expected
Mucinous Carcinoma
Clinical
♦
Ovarian mucinous carcinomas (approximately 3% of ovarian carcinomas) have a good prognosis if stage I
♦
Many older case series of ovarian mucinous carcinomas have likely contained many cases of metastatic carcinoma involving the ovary, but ovarian mucinous carcinoma, if high stage, has a poor prognosis that is worse than serous carcinoma and not significantly different from metastatic mucinous carcinoma f rom other sites involving the ovary
Macroscopic
♦
Mucinous carcino mas are usually large unilateral cystic masses filled with mucoid material
Microscopic
♦
Invasive growth pattern (“destructive stromal invasion”) or sheets of closely arranged glands with minimal or no intervening stroma must be seen for the diagnosis of ovarian mucinous carcinoma. Mucinous carcinom as are commonly seen with areas of mucinous tumor of low malignant potential and/or mucinous cystadenoma
Differential Diagnosis
♦
The primary concern when making a diagnosis of mucinous carcinoma in an ovarian specimen is the distinction of primary from metastatic tumors. Colorectal adenocarcinoma, low-grade appendiceal mucinous carcinoma, pancreatic adenocarcinoma, gastric adenocarcinoma, and cervical adenocarcinoma may all be mistaken for a primary ovarian adenocarcinoma. Primary ovarian mucinous tumors are usually limited to one ovary at presentation. Tumors which are disseminated at presentation are only rarely ovarian. The presence of bilaterality, ovarian surface involvement, and lymphovascular invasion should all raise suspicion of a metastatic carcinoma
♦




The appendix should be considered as a possible primary site for mucinous neoplasms in the ovary, but, if the appendix is grossly normal at the time of oophorectomy, routine appendectomy only rarely identifies an occult tumor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


