KEY CONCEPTS
![]() Ovarian cancer is denoted “the silent killer” because of the nonspecific signs and symptoms that contribute to the delay in diagnosis. The few patients who present with disease still confined to the ovary will have a 5-year survival rate greater than 90%, but most patients present with advanced disease and have a 5-year survival rate of 10% to 30%.
Ovarian cancer is denoted “the silent killer” because of the nonspecific signs and symptoms that contribute to the delay in diagnosis. The few patients who present with disease still confined to the ovary will have a 5-year survival rate greater than 90%, but most patients present with advanced disease and have a 5-year survival rate of 10% to 30%.
![]() Ovarian cancer is a sporadic disease with less than 10% of cases of ovarian cancer attributed to heredity. However, a history of two or more first-degree relatives with ovarian cancer increases a woman’s risk of developing ovarian cancer by greater than 50%.
Ovarian cancer is a sporadic disease with less than 10% of cases of ovarian cancer attributed to heredity. However, a history of two or more first-degree relatives with ovarian cancer increases a woman’s risk of developing ovarian cancer by greater than 50%.
![]() Considerable education efforts have been made to identify patients with the persistence, greater than 2 weeks, of nonspecific presenting symptoms of ovarian cancer including: abdominal pressure/pain, difficulty eating or feeling full quickly, urinary urgency/frequency, change in bowel habits, or unexplained vaginal bleeding.
Considerable education efforts have been made to identify patients with the persistence, greater than 2 weeks, of nonspecific presenting symptoms of ovarian cancer including: abdominal pressure/pain, difficulty eating or feeling full quickly, urinary urgency/frequency, change in bowel habits, or unexplained vaginal bleeding.
![]() CA-125 is a nonspecific antigen used as a tumor marker for diagnosis and monitoring epithelial ovarian carcinoma. If CA-125 is positive at the time of diagnosis, changes in CA-125 levels correlate with disease response and progression.
CA-125 is a nonspecific antigen used as a tumor marker for diagnosis and monitoring epithelial ovarian carcinoma. If CA-125 is positive at the time of diagnosis, changes in CA-125 levels correlate with disease response and progression.
![]() Although most patients will achieve a complete response to initial treatment, more than 50% of patients will have recurrence within the first 2 years. If recurrence is less than 6 months after completion of chemotherapy, tumor is defined to be platinum-resistant. The antitumor activity of second-line chemotherapy regimens is similar, and the choice of treatment for recurrent platinum-resistant ovarian cancer depends on residual toxicities, physician preference, and patient convenience. Participation in a clinical trial is also a reasonable option for these patients.
Although most patients will achieve a complete response to initial treatment, more than 50% of patients will have recurrence within the first 2 years. If recurrence is less than 6 months after completion of chemotherapy, tumor is defined to be platinum-resistant. The antitumor activity of second-line chemotherapy regimens is similar, and the choice of treatment for recurrent platinum-resistant ovarian cancer depends on residual toxicities, physician preference, and patient convenience. Participation in a clinical trial is also a reasonable option for these patients.
![]() Ovarian cancer is staged surgically with the International Federation of Gynecology and Obstetrics (FIGO) staging algorithm. Tumor debulking and total abdominal hysterectomy–bilateral oophorectomy surgery are the primary surgical interventions for ovarian cancer. After the completion of the staging and primary surgical treatment, the current standard of care is six cycles of a taxane/platinum-containing chemotherapy regimen.
Ovarian cancer is staged surgically with the International Federation of Gynecology and Obstetrics (FIGO) staging algorithm. Tumor debulking and total abdominal hysterectomy–bilateral oophorectomy surgery are the primary surgical interventions for ovarian cancer. After the completion of the staging and primary surgical treatment, the current standard of care is six cycles of a taxane/platinum-containing chemotherapy regimen.
![]() The interperitoneal (IP) route of chemotherapy administration has demonstrated a significant route of administration; however, it is dependent on appropriate patient selection.
The interperitoneal (IP) route of chemotherapy administration has demonstrated a significant route of administration; however, it is dependent on appropriate patient selection.
![]() A platinum-containing doublet chemotherapy regimen is the standard of care for the first recurrence of platinum-sensitive ovarian cancer.
A platinum-containing doublet chemotherapy regimen is the standard of care for the first recurrence of platinum-sensitive ovarian cancer.
![]() Despite recent advances, enrollment still primary treatment recommendation for patients with recurrent platinum-resistant ovarian cancer.
Despite recent advances, enrollment still primary treatment recommendation for patients with recurrent platinum-resistant ovarian cancer.
Ovarian cancer is a gynecologic cancer that usually arises from disruption or mutations in the epithelium of the ovary.1 It is associated with the highest mortality among the gynecologic cancers, primarily because most patients present with advanced disease. ![]() Ovarian cancer is denoted “the silent killer” because of the nonspecific signs and symptoms that often lead to a delay in diagnosis. Ovarian cancers often metastasize via the lymphatic and blood systems to the liver and/or lungs. Common complications of advanced and progressive ovarian cancer include ascites and small bowel obstruction. The few patients who present with disease still confined to the ovary will have a 5-year survival rate greater than 90%, but most patients present with advanced disease and have a 5-year survival rate of 10% to 30%. Primary treatment includes tumor-debulking surgery followed by six cycles of a taxane-platinum chemotherapy regimen. Although 70% of patients achieve an initial complete response to chemotherapy, more than 50% of these patients will have recurrence within the first 2 years from diagnosis.2
Ovarian cancer is denoted “the silent killer” because of the nonspecific signs and symptoms that often lead to a delay in diagnosis. Ovarian cancers often metastasize via the lymphatic and blood systems to the liver and/or lungs. Common complications of advanced and progressive ovarian cancer include ascites and small bowel obstruction. The few patients who present with disease still confined to the ovary will have a 5-year survival rate greater than 90%, but most patients present with advanced disease and have a 5-year survival rate of 10% to 30%. Primary treatment includes tumor-debulking surgery followed by six cycles of a taxane-platinum chemotherapy regimen. Although 70% of patients achieve an initial complete response to chemotherapy, more than 50% of these patients will have recurrence within the first 2 years from diagnosis.2
ETIOLOGY AND EPIDEMIOLOGY
It is estimated that 22,240 new cases of ovarian cancer were diagnosed, and 14,030 women died of the disease in 2013.3 Ovarian cancer is associated with the highest mortality rate among the gynecologic cancers and is the fifth leading cause of cancer-related deaths in women. Despite research efforts and recent advances, the mortality rate associated with ovarian cancer has not changed significantly over the past four decades. The high mortality rate is related to the insidious onset of nonspecific symptoms and the lack of adequate screening tools, which allows the disease to go undiagnosed until it has progressed beyond the pelvic cavity.
As with many other cancers, the risk of ovarian cancer increases with increasing age. A woman’s risk increases from 15.7 to 54 per 100,000 as her age advances from 40 to 79 years, and the median age at diagnosis is 59.3 Most cases of ovarian cancer are diagnosed during the peri- and postmenopausal phase of women’s reproductive life span.4
![]() Heredity accounts for less than 10% of all ovarian cancer cases. Family history is an important risk factor in the development of ovarian cancer. If one family member has a diagnosis of ovarian cancer, the associated lifetime risk is 9%, but this risk increases to greater than 50% if there are two or more first-degree relatives (e.g., her mother and sister) with a diagnosis of ovarian cancer or multiple cases of ovarian and breast cancer within the same family.1,2
Heredity accounts for less than 10% of all ovarian cancer cases. Family history is an important risk factor in the development of ovarian cancer. If one family member has a diagnosis of ovarian cancer, the associated lifetime risk is 9%, but this risk increases to greater than 50% if there are two or more first-degree relatives (e.g., her mother and sister) with a diagnosis of ovarian cancer or multiple cases of ovarian and breast cancer within the same family.1,2
BRCA1 and BRCA2 are the tumor suppressor genes thought to be involved in one or more pathways of DNA damage recognition and repair. The BRCA1 gene is located on chromosome 17q12–21, and the BRCA2 gene is located on chromosome 13q12–13. Both BRCA1 and BRCA2 mutations are associated with ovarian cancer. However, BRCA1 is more prevalent, being associated with 90% of inherited and 10% of sporadic cases of ovarian cancer.5 Patients with BRCA1-associated ovarian cancer are usually considerably younger than patients with BRCA2 mutations, with a mean age of 54 years.6 Patients usually present with advanced stage at diagnosis, and the BRCA1-linked ovarian cancers are more aggressive tumors that typically are serous histology, moderate to high grade. As BRCA1 and BRCA2 are thought to be involved in DNA damage or repair, their inactivation/mutations may be associated with an increased resistance of ovarian cancer cells to cytotoxic agents.
Hereditary breast and ovarian cancer syndrome is one of the two different forms of hereditary ovarian cancer and is associated with germline mutations in BRCA1 and BRCA2.5,7 The hereditary nonpolyposis colorectal cancer or Lynch syndrome is a familial syndrome with germline mutations causing defects in enzymes involved in DNA mismatch repair, which is associated with up to 12% of hereditary ovarian cancer cases.5 This syndrome is associated with mutations in DNA mismatch repair genes such as MSH2, MLH1, PMS1, and PMS2 and leads to microsatellite instability.
Hormone exposure, specifically estrogen, and reproductive history are also associated with the risk of developing ovarian cancer. Conditions that increase the total number of ovulations in women’s reproductive history, such as nulliparity, early menarche, or late menopause, are associated with an increasing risk for epithelial ovarian cancers.8,9 Conversely those conditions that limit ovulations are associated with a protective effect. Each time ovulation occurs, the ovarian epithelium is broken, followed by cellular repair. According to the incessant ovulation hypothesis, the risk of mutations and, ultimately, cancer increases each time the ovarian epithelium undergoes cell repair.
Finally, ovarian cancer is associated with certain dietary and environmental factors. A diet that is high in galactose, animal fat, and meat may increase the risk of ovarian cancer, whereas a vegetable-rich diet may decrease the risk of ovarian cancer.7,10 Although controversial, exogenous factors such as asbestos and talcum powder use in the perineal area are also associated with an increased risk of ovarian cancer.7,10
PATHOLOGY AND CLASSIFICATION
Ovarian carcinomas can be separated into three major entities: epithelial carcinomas, germ cell tumors, and stromal carcinomas. Most ovarian tumors (85% to 90%) are derived from the epithelial surface of the ovary.11 The classification of common epithelial tumors has been developed by the World Health Organization and the International Federation of Gynecology and Obstetrics (FIGO).12 The nomenclature considers cell type, location of the tumor, and the degree of the malignancy, which ranges from benign tumors to tumors of low malignancy to invasive carcinomas. Epithelial tumors classified as low malignancy (“borderline malignancy”) are characterized by epithelial papillae with atypical cell clusters, cellular stratification, nuclear atypia, and increased mitotic activity, and have a much better prognosis than those classified as invasive carcinomas. Malignant tumors are characterized by an infiltrative destructive growth pattern with malignant cells growing in a disorganized manner and dissection into stromal planes.
Invasive epithelial adenocarcinomas are characterized by histologic subtype and grade, which measures the degree of cellular differentiation. Although the histologic type of the tumor is not a significant prognostic factor, with the exception of clear cell, the histopathologic grade is an important prognostic factor. Undifferentiated tumors are associated with a poorer prognosis than those lesions that are considered to be well or moderately differentiated. A universal grading system for ovarian cancer was developed that combines mitotic score, nuclear atypia score, and architectural score based on the histologic pattern.13
The histologic subtypes of adenocarcinomas include papillary serous, mucinous, endometrioid, clear cell, mixed epithelial, transition-cell, and undifferentiated.2,4,13 Papillary serous adenocarcinoma is the most common type of epithelial ovarian cancer and accounts for about 46% of cases. The peak age of diagnosis ranges from 45 to 65 years with 63 years as the median age of diagnosis.14 Serous carcinomas typically display complex papillary and solid patterns and qualify as high-grade carcinomas. Endometrioid carcinomas are seen in women 40 to 50 years of age and comprise about 8% of ovarian carcinomas, of which about 6% are surface epithelial neoplasms.14 Endometrioid tumors are usually diagnosed as stage I disease and have a better prognosis than tumors with serous histology. Mucinous carcinomas occur in women between 40 and 70 years of age and account for about 36% of all ovarian cancers. The overall prognosis for mucinous carcinoma is better than for serous carcinoma because most patients present with stage I disease. Clear cell carcinoma comprises about 3% of ovarian carcinomas in women, with a mean age of 57 years. Although clear cell carcinoma is the least common ovarian neoplasm, it is most commonly associated with paraneoplastic-related hypercalcemia.14
Germ cell tumors of the ovary, including malignant teratoma and dysgerminomas, are rare, comprising about 2% to 3% of all ovarian cancers in Western countries with an increased incidence in black and Asian women.15,16 These tumors are highly curable and affect primarily young women. In contrast to epithelial tumors, about 60% to 70% of germ cell tumors are stage I at diagnosis, which is related to earlier detection and response to symptoms in this younger patient population.16 Serum markers (human β-chorionic gonadotropin and α-fetoprotein) are helpful to confirm the diagnosis and monitor response to treatment.
Finally, ovarian sex cord-stromal tumors account for 7% of all ovarian cancers and tend to be diagnosed at stage I.12 Sex cord-stromal tumors are associated with hormonal effects, such as precocious puberty, amenorrhea, and postmenopausal bleeding. Because these tumors are rare, the optimal treatment of ovarian sex cord-stromal tumors is not clear. The current recommended standard of care is surgery followed by treatment with a platinum-based chemotherapy regimen.
Ovarian cancer is usually confined to the abdominal cavity, but spread can occur to the lung, liver, and, less commonly, the bone or brain. Disease is spread by direct extension, peritoneal seeding, lymphatic dissemination, or bloodborne metastasis. Lymphatic seeding is the most common pathway and frequently causes ascites.
SCREENING AND PREVENTION
Screening
Ovarian cancer is an uncommon disease with no known preinvasive component, which has made it difficult to screen patients to detect early disease. In addition, the risk factors for developing ovarian cancer are not well understood, which also makes it difficult to identify a high-risk group of individuals. At the present time, there are no effective screening tools for early detection of ovarian cancer. ![]() However, considerable education efforts have been made to help identify patients with the persistence (i.e., >2 weeks) of nonspecific presenting symptoms of ovarian cancer including: abdominal pressure/pain, difficulty eating or feeling full quickly, urinary urgency/frequency, change in bowel habits, or unexplained vaginal bleeding.
However, considerable education efforts have been made to help identify patients with the persistence (i.e., >2 weeks) of nonspecific presenting symptoms of ovarian cancer including: abdominal pressure/pain, difficulty eating or feeling full quickly, urinary urgency/frequency, change in bowel habits, or unexplained vaginal bleeding.
Pelvic examinations are noninvasive and well accepted and can detect large tumors with a sensitivity of 67% for detecting all tumors.15 However, because pelvic examinations cannot detect minimal or microscopic disease, they do not usually detect ovarian cancer until it is in an advanced stage. As a result of these limitations, routine pelvic examinations are not an effective screening tool and do not decrease overall mortality.15
Transvaginal ultrasound (TVUS) creates an image of the ovary by releasing sound waves. It can be used to evaluate the size and shape and to detect the presence of cystic or solid masses or abdominal fluid. TVUS can also evaluate blood flow within an ovarian mass. Normal ovarian size cutoff parameters range from 1.25 cm2 for women 55 to 59 years of age to 1.0 cm2 for women older than age 65 to 69 years.17,18 TVUS is sensitive in identifying ovarian lesions and abnormalities, but its use as a routine screening test is limited by a lack of specificity and an inability to detect peritoneal cancer or cancer in normal-size ovaries.19,20
Serum cancer antigen-125 (CA-125) is a nonspecific inflammatory antigen that can be elevated in numerous conditions associated with inflammation in the abdominal cavity. CA-125 has been extensively studied as a potential tumor marker for ovarian cancer based on the observation that CA-125 levels in a woman without ovarian cancer tend to stay the same or decrease over time, whereas levels associated with malignancy tend to gradually increase over time.19 However, CA-125 is a nonspecific test that can be elevated in a number of benign conditions, including other gynecologic conditions, such as endometriosis, and many nongynecologic conditions, such as diverticulitis and peptic ulcer disease. Because of these limitations, CA-125 levels are not recommended as a routine screening test for detection of ovarian cancer. Numerous other serologic markers such as carcinoembryonic antigen and lipid-associated sialic acid have been evaluated but cannot be recommended for routine screening for ovarian cancer.
The United States Preventive Services Task Force found fair evidence to support screening with CA-125 or TVUS and concluded that earlier detection would likely have a small effect, at best, on mortality from ovarian cancer.21 Unfortunately, because of the low prevalence of ovarian cancer and the invasive nature of diagnostic testing after a positive screening test, the United States Preventive Services Task Force also found fair evidence that screening could likely lead to important harms. The United States Preventive Services Task Force concluded that the potential harms outweigh the potential benefits and recommended against any form of routine screening with CA-125 or TVUS for ovarian cancer.
In high-risk women, as defined by family history, most clinicians use a multimodality approach for ovarian cancer screening that includes an annual TVUS in combination with a CA-125 blood test every 6 months. Changes in CA-125 are monitored over time, and changes such as a persistent elevation or consistent increases in CA-125 levels in conjunction with TVUS abnormalities are evaluated further.
Prevention
It is difficult to make recommendations for prevention for the general population because ovarian cancer is a sporadic disease with no established risk factors. Noninvasive measures, such as chemoprevention, have demonstrated some benefit in decreasing the risk of developing ovarian cancer. Ovulation itself is considered a potential insult to the ovarian epithelium, increasing its susceptibility to damage and, ultimately, to cancer. Interventions or reproductive conditions associated with decreasing the number of ovulations, including multiparity, may have a protective effect for the prevention of ovarian cancer. However, the more invasive prevention interventions, such as prophylactic surgery and genetic screening, should be reserved for those women identified to be at high risk based on their heredity for developing ovarian cancer.
Chemoprevention
Although a number of agents have been investigated as chemoprevention of ovarian cancer, including oral contraceptives, aspirin, nonsteroidal antiinflammatory agents, and retinoids, none of these agents is currently accepted as standard treatment for the prevention of ovarian cancer. Oral contraceptives inhibit ovulation, which reduces the opportunity for potential for damage to the ovarian epithelium. When taken for longer than 10 years, oral contraceptives decrease the relative risk to less than 0.4.22,23 Because oral contraceptive use is associated with an increased risk of breast cancer, women with a family history of breast cancer are not candidates for this use of oral contraceptives as chemoprevention of ovarian cancer.22,23
Nonsteroidal antiinflammatory drugs, aspirin, and acetaminophen also have been suggested for use in the chemoprevention of different cancers, especially hereditary nonpolyposis colon cancer.24 Although the results of observational studies show that the use of nonsteroidal antiinflammatory drugs, aspirin, and acetaminophen reduces the risk of ovarian cancer, these findings have not been confirmed in prospective clinical studies. The proposed mechanism of these agents is the antiinflammatory effect on normal ovulation and inhibition of ovulation.24,25
Prophylactic Surgery
Prophylactic surgical interventions for the prevention of ovarian cancer are reserved for patients with a significant family history and/or with known genetic mutations such as BRCA1 and should be postponed until after childbearing is completed. The goal is to remove healthy, at-risk organs before any carcinogenic activity is initiated, ultimately reducing the risk of developing cancer. These surgeries include prophylactic oophorectomy or bilateral salpingo-oophorectomy and tubal ligation. These procedures will cause surgical menopause, which can be associated with severe hot flashes, vaginal dryness, sexual dysfunction, and increased risk for development of osteoporosis and heart disease in these women. Because of the potential impact on quality of life and increased health risks, prophylactic surgery is not recommended as a general prevention intervention for the general population.
Although prophylactic surgical interventions are associated with significant reduction in risk of developing ovarian cancer, patients who choose to have a prophylactic oophorectomy/bilateral salpingo-oophorectomy completed need to be informed that complete protection is not guaranteed.15,23,26 Although a 67% risk reduction has been shown, a potential 2% to 5% risk of primary peritoneal cancer remains.27,28 Primary peritoneal cancers have identical histology of ovarian tumors with diffuse involvement of peritoneal surfaces. Primary peritoneal cancers can often result from “seeding” during the prophylactic surgery. It is recommended for peritoneal washings to be completed during the prophylactic surgery to check for presence of peritoneal surfaces. If positive, then prophylactic surgery would change to staging and treatment surgery to determine extent of disease and remove any other possible lesions.
Tubal ligation is another procedure that can potentially reduce the risk for developing ovarian cancer. In a case-control study, Narod et al. reported that tubal ligation in BRCA-positive women was associated with a 63% reduction in risk of developing ovarian cancer.29 However, it is not recommended as a sole procedure in prophylaxis. The mechanism for its protective effect is not clear, but it has been proposed that tubal ligation may limit exposure of the ovary to environmental carcinogens.
Genetic Screening
Genetic screening should be considered for those women with a significant family history of ovarian cancer. Patients should be evaluated for the presence of genes such as BRCA1, BRCA2, or other genes such as those associated with hereditary nonpolyposis colorectal cancer or the hereditary breast ovarian cancer (hereditary breast and ovarian cancer syndrome) syndrome.29–32 Prior to genetic screening, appropriate patient/family counseling and genetic counseling should be available to help women prepare and deal with the health and psychosocial implications of the genetic screening results.
CLINICAL PRESENTATION
CLINICAL PRESENTATION
Patients with early ovarian cancer are often asymptomatic and the ovarian mass is often detected incidentally during their annual pelvic examinations. Patients with ovarian cancer often present with nonspecific, vague symptoms such as abdominal bloating, pressure or pain, indigestion, or change in bowel movements.2,4,33 These symptoms can easily be confused with symptoms of common benign gastrointestinal disorders. Patients will often not seek medical attention until these symptoms become unrelenting and bothersome, which allows the disease to progress undetected. Patients with advanced disease may report symptoms such as pain, abdominal distension, and ascites.2,33
Several groups have partnered together to educate women about early signs and symptoms of ovarian cancer. Goff et al. recently developed a symptom index, based on a comparison of symptoms experienced in patients with ovarian cancer and a matched control group.34 Symptoms that were correlated with ovarian cancer were persistent or recurrent bloating, pelvic or abdominal pain, difficulty eating or feeling full quickly, and urinary symptoms (either urgency or frequency). The Gynecologic Cancer Foundation, Society of Gynecologic Oncologists, and American Cancer Society recommend that women who have any of those problems nearly every day for more than 2 weeks should see a gynecologist, especially if the symptoms are new and quite different from her usual state of health. Furthermore, healthcare professionals should keep ovarian cancer in the differential for women presenting with these persistent symptoms.
DIAGNOSIS
The diagnostic workup for suspected ovarian cancer includes a careful physical examination including a Papanicolaou (Pap) smear and a pelvic and rectovaginal examination.7 The presence of a pelvic mass that is unilateral or bilateral, solid, irregular, fixed, or nodular is highly suggestive of ovarian cancer. Unfortunately, by the time a pelvic mass can be palpitated on physical exam, the disease is already advanced beyond the pelvic cavity. A detailed family history should be taken, especially noting the number and pattern of first-degree relatives with malignancies.
A complete blood count, chemistry profile (including liver and renal function tests), and CA-125, carcinoembryonic antigen, and CA19–9 levels should be performed. ![]() Although CA-125 is a nonspecific antigen, it is the best current tumor marker for epithelial ovarian carcinoma. A normal CA-125 value is less then 35 units/mL (35 kU/L). If CA-125 is elevated at the time of diagnosis, changes in CA-125 levels correlate with tumor burden. Rising CA-125 levels are often associated with disease progression, but CA-125 can be elevated in various other conditions such as different phases of the menstrual cycle, diverticulitis, endometriosis, as well as other nongynecologic cancers. When a patient presents with an abdominal mass, it is important to rule out other cancers in the abdominal cavity. Carcinoembryonic antigen and CA19–9 are markers for other gastrointestinal cancers and may be helpful in the differential diagnosis.
Although CA-125 is a nonspecific antigen, it is the best current tumor marker for epithelial ovarian carcinoma. A normal CA-125 value is less then 35 units/mL (35 kU/L). If CA-125 is elevated at the time of diagnosis, changes in CA-125 levels correlate with tumor burden. Rising CA-125 levels are often associated with disease progression, but CA-125 can be elevated in various other conditions such as different phases of the menstrual cycle, diverticulitis, endometriosis, as well as other nongynecologic cancers. When a patient presents with an abdominal mass, it is important to rule out other cancers in the abdominal cavity. Carcinoembryonic antigen and CA19–9 are markers for other gastrointestinal cancers and may be helpful in the differential diagnosis.
Other diagnostic tests should include a transvaginal or abdominal ultrasonography, chest radiography, computed tomography, magnetic resonance imaging, or positron emission tomography scan. An upper GI series, IV pyelogram, cystoscopy, proctoscopy, or barium enema is sometimes indicated to confirm diagnosis and extent of disease.
TREATMENT
Ovarian Cancer
Desired Outcomes
The goals of treatment of ovarian cancer are dependent upon the FIGO stage at diagnosis. While ideally “treatment for cure” is desired, it is important to set realistic expectations for the patient. ![]() Most patients will achieve a complete response to the initial multimodality treatment, but over 50% of these patients will present with recurrent disease within the first 2 years after completion of treatment. Although overall survival has not significantly changed for ovarian cancer patients, the progression-free survival has improved, which translates to less time on chemotherapy and overall improvement in quality of life for these patients.
Most patients will achieve a complete response to the initial multimodality treatment, but over 50% of these patients will present with recurrent disease within the first 2 years after completion of treatment. Although overall survival has not significantly changed for ovarian cancer patients, the progression-free survival has improved, which translates to less time on chemotherapy and overall improvement in quality of life for these patients.
In patients who present with metastatic disease or are not surgical candidates, the goal of treatment becomes focused on alleviating symptoms and prolonging survival as long as quality of life is acceptable. In the setting of recurrent platinum-resistant ovarian cancer, the treatment goal is also focused on alleviating symptoms and prolonging survival as long as quality of life is acceptable. ![]() Phase I/investigational agent clinical trials should be considered because they may be the best treatment option for potential benefit due to the poor efficacy of the available chemotherapy agents for recurrent platinum-resistant ovarian cancer.
Phase I/investigational agent clinical trials should be considered because they may be the best treatment option for potential benefit due to the poor efficacy of the available chemotherapy agents for recurrent platinum-resistant ovarian cancer.
General Approach
![]() A multimodality approach that includes comprehensive surgery and chemotherapy is used for the initial treatment of ovarian cancer with curative intent. Although most patients will initially achieve a complete response, more than 50% will recur within the first 2 years.2,35 A clinical complete response to treatment is defined as no evidence of disease by physical examination or diagnostic tests and a normal CA-125 level.
A multimodality approach that includes comprehensive surgery and chemotherapy is used for the initial treatment of ovarian cancer with curative intent. Although most patients will initially achieve a complete response, more than 50% will recur within the first 2 years.2,35 A clinical complete response to treatment is defined as no evidence of disease by physical examination or diagnostic tests and a normal CA-125 level.
Chemotherapy regimens for ovarian cancer have evolved over the past several decades. Treatment regimens began with single-agent melphalan followed by single-agent cyclophosphamide. Shortly after cisplatin was introduced into clinical practice, it was added to cyclophosphamide, and this combination was the “standard of care” for more than a decade until the introduction of paclitaxel in the 1980s. Paclitaxel soon replaced cyclophosphamide, and paclitaxel plus cisplatin became the standard of care. Carboplatin was then substituted for cisplatin because of its improved toxicity profile, and paclitaxel plus carboplatin was adopted. During this same period, many researchers have conducted numerous clinical trials of intraperitoneal (IP) chemotherapy. In 2006, Armstrong and colleagues published the first IP therapy clinical trial to demonstrate a survival advantage over the standard IV regimen.36 However, these advances in chemotherapy for the treatment of ovarian cancer have not yet translated into major changes in overall 5-year survival, which remains less than 20%.
Certain subgroups of patients have a better or worse response to chemotherapy. The histologic subtype of the tumor is a prognostic factor; clear cell histology is more likely to be poorly differentiated, faster growing, and have intrinsic drug resistance.2,37 However, the extent of residual disease, size larger than 1 cm, and tumor grade are better predictors of response to chemotherapy and overall survival.2
In general, younger patients have a better performance status and tolerate chemotherapy better than elderly patients. For unknown reasons, white women tend to have a worse prognosis and response to therapy as compared with women of other ethnic backgrounds.2,6,7
In patients with recurrent ovarian cancer, the goals of treatment are to relieve symptoms such as pain or discomfort from ascites, slow disease progression, and prevent serious complications such as small bowel obstructions.
Surgery
Surgery is the primary treatment intervention for ovarian cancer.37–41 Surgery may be curative for selected patients with limited stage IA disease.
Primary surgical treatment includes a total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH/BSO), omentectomy, and lymph node dissection (Fig. 110-1).37–41 The primary objective of the surgery is to optimally debulk the tumor to less than 1 cm of residual disease.42 Long-term followup studies confirm that residual disease smaller than 1 cm correlates with higher complete response rates to chemotherapy and longer overall survival as compared to patients with bulky residual disease (>1 cm).40,41
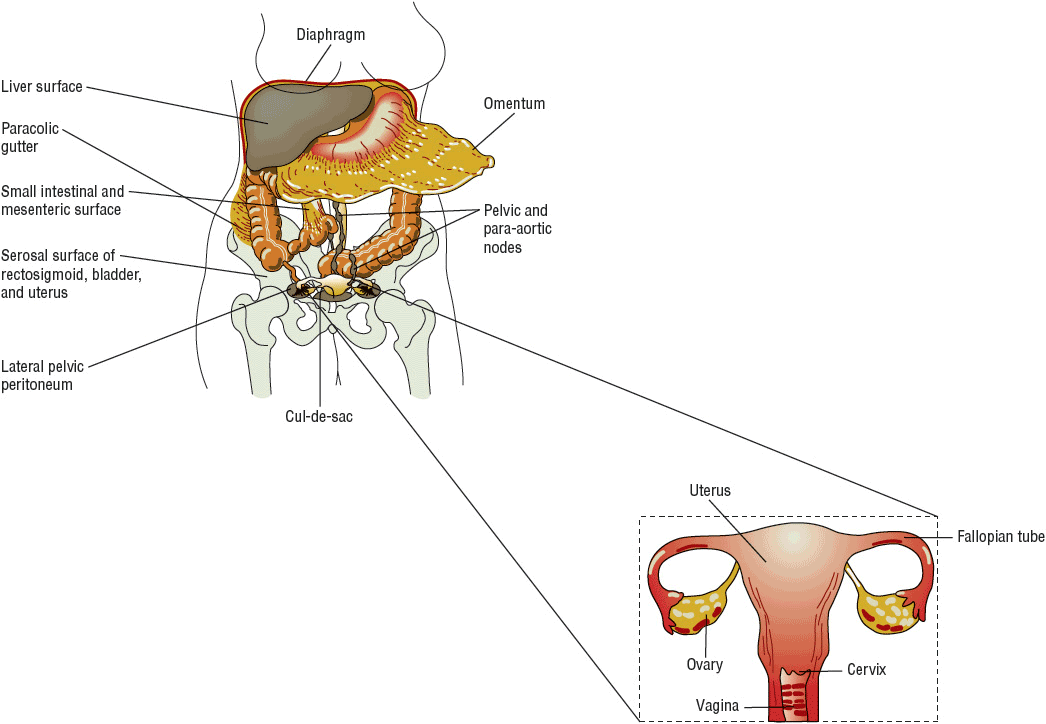
FIGURE 110-1 Staging laparotomy for ovarian cancer with diagram of female reproductive tract (uterus, fallopian tubes, ovaries, vagina). Dashed line box outlines what is removed during the total abdominal hysterectomy with bilateral salpingo-oophorectomy.
A comprehensive exploratory laparotomy is vital for the accurate confirmation of diagnosis and staging of ovarian cancer.37–39 ![]() Unlike other cancers that are typically diagnosed by biopsy or laboratory results and clinically staged by results from imaging tests, gynecologic cancers, such as ovarian cancer, are surgically diagnosed and then staged according to the FIGO staging algorithm (Fig. 110-2). The FIGO staging system requires a fairly extensive surgery by an experienced gynecologic oncologist. The skill of the surgeon has a significant effect on prognosis, with definitive benefit of a trained gynecologic oncologist performing surgery as compared with a gynecologist or general surgeon.43 The reasons for this approach include (a) pelvic tumors cannot be readily biopsied without risk of “tumor seeding,” which can increase the risk of recurrence, and (b) surgical staging takes into account the presence of microscopic disease in samples obtained by pelvic washing and lymph node dissection and read by a pathologist during the surgical procedure. It is recommended that the initial surgical staging and tumor-debulking surgery be completed by a trained gynecologic oncology surgeon when ovarian cancer is suspected to prevent understaging and to optimize overall outcome.44
Unlike other cancers that are typically diagnosed by biopsy or laboratory results and clinically staged by results from imaging tests, gynecologic cancers, such as ovarian cancer, are surgically diagnosed and then staged according to the FIGO staging algorithm (Fig. 110-2). The FIGO staging system requires a fairly extensive surgery by an experienced gynecologic oncologist. The skill of the surgeon has a significant effect on prognosis, with definitive benefit of a trained gynecologic oncologist performing surgery as compared with a gynecologist or general surgeon.43 The reasons for this approach include (a) pelvic tumors cannot be readily biopsied without risk of “tumor seeding,” which can increase the risk of recurrence, and (b) surgical staging takes into account the presence of microscopic disease in samples obtained by pelvic washing and lymph node dissection and read by a pathologist during the surgical procedure. It is recommended that the initial surgical staging and tumor-debulking surgery be completed by a trained gynecologic oncology surgeon when ovarian cancer is suspected to prevent understaging and to optimize overall outcome.44
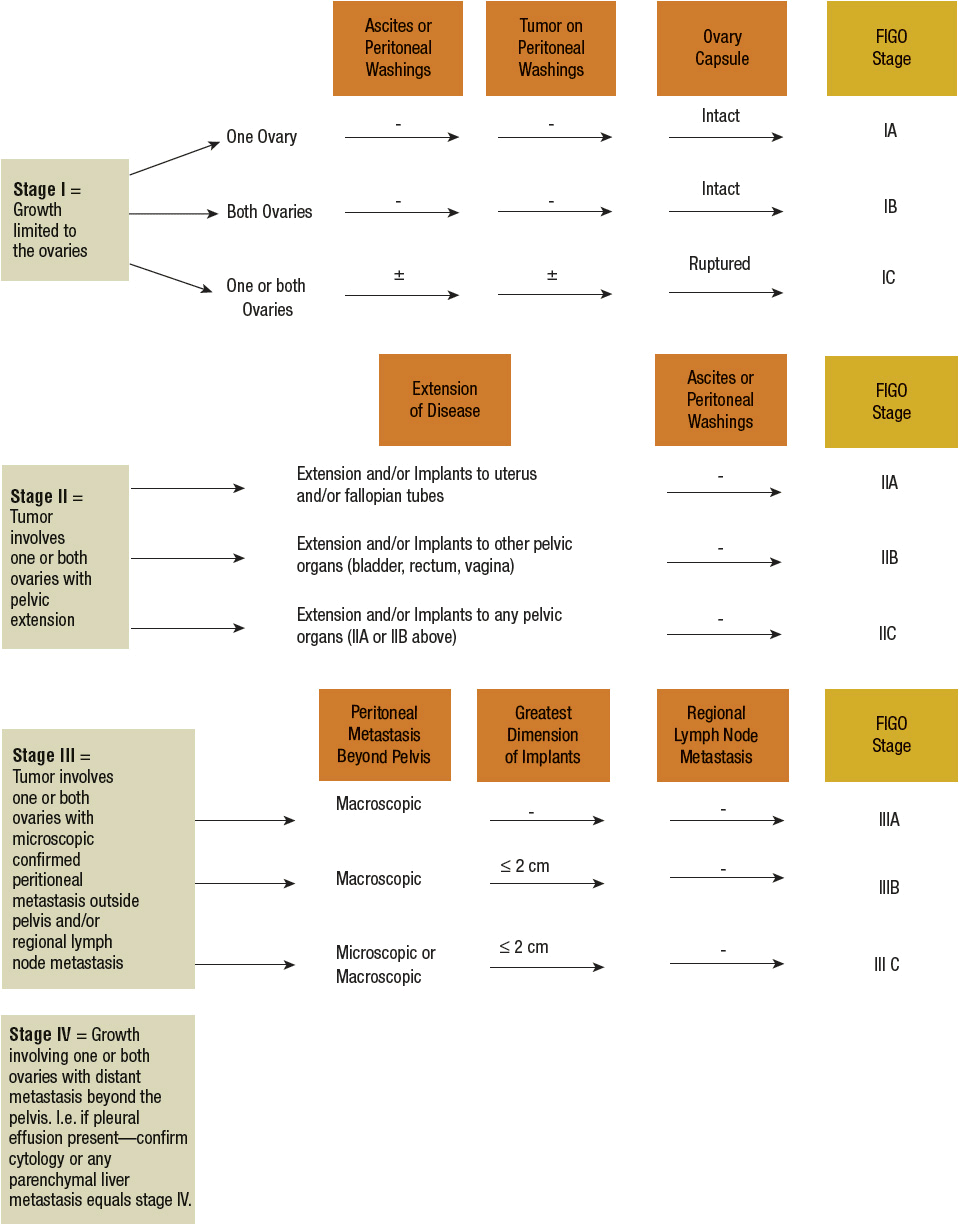
FIGURE 110-2 International Federation of Gynecology and Obstetrics (FIGO) staging algorithm.
Secondary cytoreduction or interval debulking is when surgery is performed after completion of some or all chemotherapy to remove residual disease. Some protocols include additional cycles of chemotherapy after the surgical procedure. The importance of cytoreduction before, during, or after chemotherapy is still controversial, but it has been recommended to facilitate response to chemotherapy and improve overall survival. Randomized trials of secondary surgical cytoreduction have reported conflicting results. In an older randomized trial, van der Burg et al. performed interval debulking surgery on 140 stage IIB to stage IV suboptimally debulked (<1 cm of residual disease) ovarian cancer patients after receiving three cycles of cisplatin plus cyclophosphamide.45 Patients then received an additional three cycles of these same drugs after surgery. Patients randomized to the nonsurgical treatment arm received six cycles of chemotherapy. Interval debulking surgery significantly prolonged overall and progression-free survival and reduced the risk of death by 33%. However, in a recently published study of 550 women with stage III or IV disease treated with primary cytoreductive surgery and three cycles of paclitaxel and cisplatin, patients randomized to receive secondary cytoreductive surgery followed by three more cycles of chemotherapy had similar progression-free survival and overall survival as compared with those randomized to receive three more cycles of chemotherapy alone.46
The overall effect of interval debulking is influenced by several factors, including initial response to chemotherapy, the amount of residual disease before and after second-look surgery, and the presence of microscopic residual disease. The results of recent trials suggest that secondary surgical cytoreduction does not prolong survival in patients who are treated with maximal primary cytoreductive surgery followed by appropriate postoperative chemotherapy.
“Second-look surgery” is an elective surgical procedure performed in patients who achieve a clinical complete response after primary chemotherapy to determine if any visible or microscopic disease is present in the peritoneal cavity. The benefit of “second-look laparotomy” to evaluate residual disease after completing chemotherapy remains controversial because it has been difficult to establish any impact on overall survival. It has questionable benefit because approximately 50% of those with a negative second look still relapsed.3 If visible or microscopic disease is detected during second look, then the clinician may decide to give additional chemotherapy. But if no visible or microscopic disease is detected during second look, the clinician may decide to observe and monitor the patient. Use of laparoscopic surgical techniques is controversial for initial surgery but is sometimes considered in debulking of recurrent or advanced disease when the intent is palliative rather than curative.40 In patients with recurrent disease, the goal of debulking surgery is to relieve symptoms associated with complications such as small bowel obstructions and to help improve the patient’s quality of life.
Radiation
Radiation has a limited role in the management of ovarian cancer. Use of radiation for treatment of early stage disease has had no benefit or impact on overall survival.47 Radiation therapy is most beneficial for palliation of symptoms in patients with recurrent pelvic disease, often associated with small bowel obstructions. The two forms of radiation therapy used in ovarian cancer are external beam whole-abdominal irradiation and intraperitoneal isotopes such as phosphorus-32 (32P). Alleviation of symptoms with external beam whole-abdominal irradiation is associated with a significant improvement in the patient’s quality of life. The recommended dose ranges from 35 to 45 Gy (3500 to 4500 rad), depending on the treatment history and ability to tolerate radiation treatments.
First-Line Chemotherapy
The mainstay of ovarian cancer treatment is chemotherapy. It is used as a component of first-line treatment after completion of surgery and is the primary modality of treatment for recurrent ovarian cancer.
Systemic chemotherapy with a taxane and platinum regimen following optimal surgical debulking is the standard of care for treatment of epithelial ovarian cancer (Fig. 110-3). Table 110-1 summarizes the chemotherapeutic regimens used as the initial treatment of newly diagnosed epithelial ovarian cancer. More than 60 randomized, controlled clinical trials have evaluated combination chemotherapy regimens for the treatment of advanced ovarian cancer, and a meta-analysis of these trials confirmed the efficacy of platinum and taxane regimens over other regimens.48,49
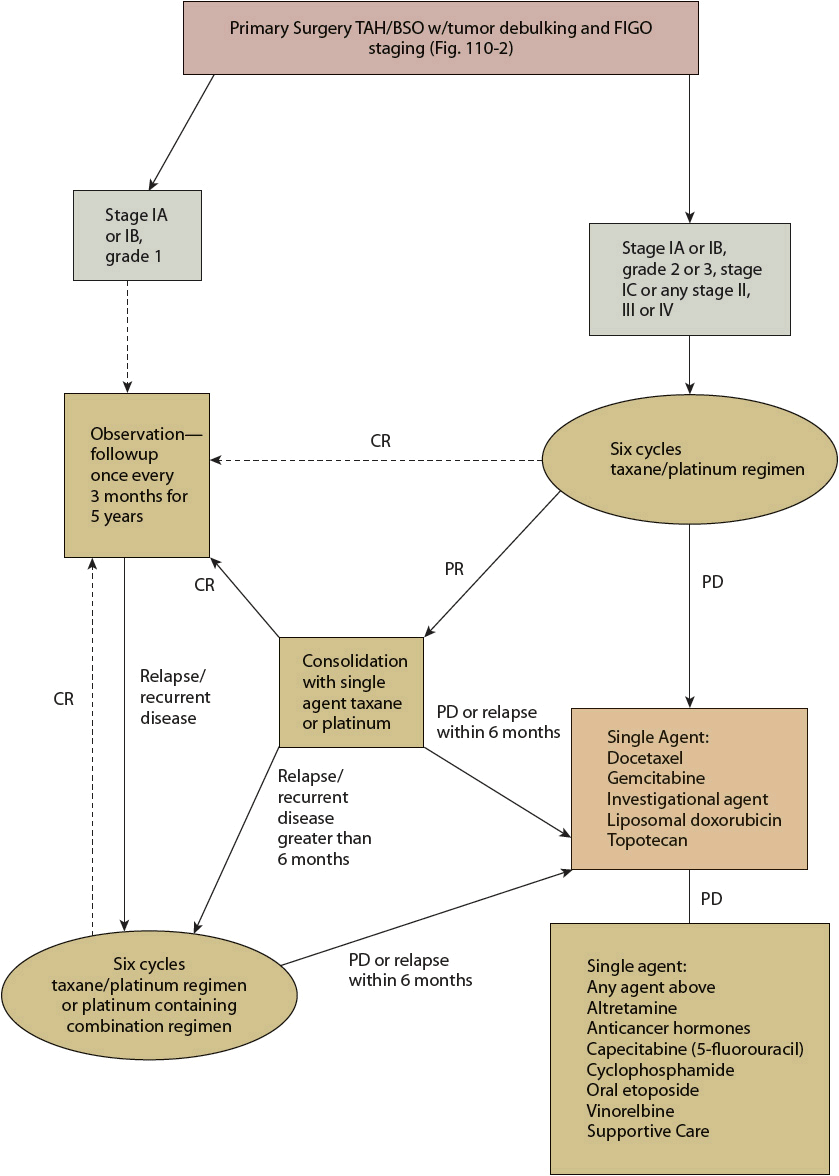
FIGURE 110-3 Management of newly diagnosed, refractory, and progressive epithelial ovarian cancer. All recommendations are category 2A unless otherwise indicated. (CR, complete response; PD, progression of disease; PR, partial response; TAH/BSO, total abdominal hysterectomy/bilateral salpingo-oophorectomy; USO, unilateral salpingo-oophorectomy.)



