INTRODUCTION
From hearing loss or nasal hemorrhage (epistaxis) to endocrine surgery and expert management of acute airway emergencies, otolaryngology/head and neck surgery is a surgical subspecialty which focuses on the management of a wide range of disorders of the head and neck. As a colleague once put it, “Otolaryngology pretty much covers everything above the clavicles except the eyes, the brain, and the spinal cord.” The limits inherent to a single book chapter preclude covering such a broad field comprehensively. Therefore this chapter will present an overview of selected disease processes in otolaryngology that are of importance to the general surgeon in training. Other important parts of otolaryngology such as surgical endocrine disorders of the neck (eg, thyroid and parathyroid), facial plastic and reconstructive surgery, and facial skeletal trauma are covered in separate chapters.
DISORDERS OF THE EAR, AUDITORY, VESTIBULAR SYSTEMS, AND TEMPORAL BONE
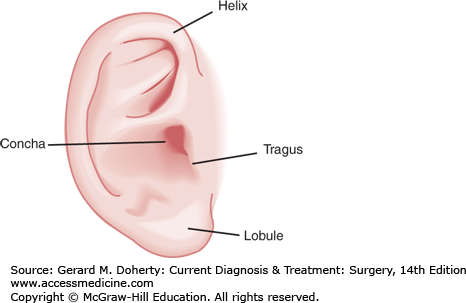
The external ear consists of two parts, the auricle (projecting from the lateral aspect of the head) and the external auditory canal (EAC) projecting medially to the tympanic membrane. Functioning as resonant amplifiers of sound energy, the concha of the auricle (Figure 15–1) has a resonance frequency of approximately 5 KHz, and the EAC has a resonance frequency of approximately 3.5 KHz. Combined, the external ear amplifies sound by approximately 10-15 dB in the 2-5 KHz range.
The tympanic membrane is positioned in an oblique plane, separating the EAC from the middle ear. It functions in transforming acoustic energy from sound waves to mechanical energy, which is transmitted via the ossicles—malleus, incus, and stapes to the oval window of the cochlea. The mechanics of the middle ear further amplify sound energy using two methods. First, the tympanic membrane is approximately 17 times larger than the footplate of the stapes; second, the ossicles act as a lever, providing a mechanical advantage of 1:1.3 from the tympanic membrane to the oval window. Combined, the result in a 25-30 dB gain in amplification.
The temporal bone houses the bony portion of the EAC, the middle, and the inner ear. The otic capsule of the inner ear is the hardest bone in the human body. Other important structures passing through or adjacent to the temporal bone include the carotid artery, the jugular vein, and the facial nerve (seventh cranial nerve). All of these structures are at risk for injury from temporal bone trauma.
The inner ear consists of the cochlea, which is both the auditory and vestibular sense organ. The vestibular system senses both linear acceleration (gravity) and angular acceleration (rotation). The hearing portion of the cochlea is a coiled tube that resembles a snail. Divided into three separate chambers, the scala vestibule and the scala tympani are filled with perilymph (similar in composition to extracellular fluid), while the scala media is filled with endolymph (similar in composition to intracellular fluid). The endolymph composition is maintained by Na+/K+ ATPase pumps within the stria vascularis found on the lateral walls of the scala media. These different chambers thus have different electrolyte composition, creating an electrical potential between the compartments. The sound energy, once transferred through the ossicles to the oval window of the cochlea, is coupled directly to the perilymph of the scala vestibule.
The resulting traveling wave is in the form of mechanical energy, which is then converted to electrical (neural) impulses within the scala media by the organ of Corti. The organ of Corti consists of inner hair cells (which are the sensory cells) and outer hair cells (which function as modulators of the inner hair cells), support cells and tectorial membrane. These nerve impulses produced by the inner hair cells are transmitted to the brainstem by the eighth cranial nerve, which travels from the cochlea through the internal auditory canal (IAC).
The vestibular system consists of the utricle, saccule, and three semicircular canals. Enveloped within the endolymphatic membrane, which is filled with endolymph, they are surrounded by perilymph, and then the very hard bone of the otic capsule. The utricle detects horizontal acceleration while the saccule detects vertical acceleration. Three semicircular canals, situated at right angles to each other, and paired with a semicircular canal on the opposite side of the head, detect angular acceleration.
Simplified, linear or angular motion causes their respective sensory cells to deflect, and the sensory cell body to depolarize. Depending on the direction, each vestibular apparatus will either increase or decrease the discharge rate relative to the basal rate, providing both direction, and speed of acceleration. Vestibular information is transmitted to the brainstem by the vestibular branch of the eighth cranial nerve.
The sudden onset of unilateral (or more uncommonly bilateral) hearing loss occurs at an annual incidence of 5-20 cases per 100,000 persons and can be an extremely unsettling experience for a patient. Most experts use a definition based on at least a 30 dB hearing loss occurring over 3 days or less. The causes of SSHL include viral infection (particularly herpes-family viruses), trauma, vascular compromise from thromboembolic phenomena or vasospasm, autoimmune disease, ototoxic (from chemotherapy, antibiotics, or salicylates), and congenital anatomic defects.
Prompt evaluation and initiation of treatment is critical, and may improve the prognosis for hearing improvement. Because of the multitude of potential mechanisms, a careful history should be performed. Often, the cause remains unknown. Patients may report an antecedent loud noise exposure such as an explosion (suggesting traumatic perilymphatic fistula), or upper respiratory tract infection symptoms (suggesting possible viral mechanism). Recent heart surgery or thromboembolic phenomenon could suggest a vascular etiology. Patients should be questioned about coincident imbalance or vertiginous symptoms, and tinnitus (ringing in the ears), indicative of vestibular as well as auditory pathology.
Formal pure-tone audiometry testing should be obtained. If the patient reports vestibular or balance symptoms, investigation of the vestibular system should be performed. Dix–Hallpike and Baranay maneuvers test for vertigo in response to specific position changes with respect to gravity. Simple tests of the auditory system (Weber and Rinne tests) can also be performed at the bedside using a 512 KHz tuning fork (Table 15–1). Pneumatic otoscopy should be performed, to evaluate for fistula sign (vertigo on pneumotoscopy insufflation).
Workup should include evaluation for a vestibular schwannoma, as this represents approximately 1%-3% of the sudden sensorineural hearing loss (SSHL). This can be done by the auditory evoked brainstem response (ABR), or a magnetic resonance imaging (MRI) with contrast using IAC protocols. Some experts advocate a less expensive screening MRI without contrast as an acceptable first step, followed by more definitive evaluations if any abnormalities are present. Laboratory studies should include a CBC with differential, ESR, PT/PTT, and cochlear antibodies. Additional studies that may be helpful include syphilis testing (either MHA-TP or FTA-Abs), and thyroid function tests. If there is a family history of sudden hearing loss, a computed tomography (CT) scan looking for enlarged vestibular aqueduct can be obtained.
If the workup reveals a cause for the SSHL, this should be addressed. Unfortunately, the majority remain idiopathic. In this case, unless contraindicated due to comorbidities, initial treatment should be started empirically. Oral corticosteroids, such as a prednisone taper (60 mg daily for 9 days then tapering over 5 days), and antiviral medication (such as acyclovir) should be administered for a minimum of 2 weeks. Other treatments such as hyperbaric oxygen, carbogen inhalation, anticoagulation, and diuretics have been proposed for the treatment of SSHL but results of these therapies have been inconclusive.
The prognosis for spontaneous recovery of function is hopeful, and approximately 60% of patients will recover full or partial hearing. With corticosteroid treatment, this may increase to 80% according to some studies. The addition of acyclovir has not been shown to be effective, but some experts recommend it due to low side effects and a plausible mechanism of action.
The seventh cranial nerve (facial nerve) innervates the muscles of facial expression (as well as special motor afferents to the parotid and lacrimal glands). A careful history will help define the onset of paralysis (eg, acute deterioration over less than 2-3 days, or gradual decline). Antecedent events (temporal bone trauma, acute otitis media [AOM], hearing loss or imbalance, and recent viral illness) should be elicited and can help guide further workup and management.
Potential causes of acute facial nerve paralysis are numerous; however over 50% are idiopathic and termed “Bell Palsy.” Note that Bell Palsy is a diagnosis of exclusion, and therefore a focused but complete workup must be performed. Fully 20% of facial nerve paralyses are caused by trauma. Other common causes (but by no means an inclusive list) include herpes zoster oticus (Ramsey Hunt syndrome), complications from otitis media and mastoiditis, Lyme disease, cholesteatoma, and neoplasm.
A complete physical examination with emphasis on the neurologic system and cranial nerves is critical. An important consideration in evaluating the patient with acute facial nerve paralysis is distinguishing between central and peripheral lesions. In central lesions, there is sparing of forehead elevation on the affected side due to decussating fibers from the contralateral side. In peripheral lesions the fibers have already crossed and there is no forehead sparing. The degree of facial nerve functional loss should be documented and has important bearing on prognosis. Several grading systems have been proposed; the House–Brackmann scale is the most widely used and is presented in Table 15–2. Facial nerve function is graded from I (normal) to VI (total paralysis) for each side.
| Grade | Characteristics |
|---|---|
| I (Normal) | Normal function in all branches |
| II (Mild dysfunction) | Slight weakness to visual inspection Normal symmetry and tone at rest Forehead: moderate to good function Eye: complete closure, minimal effort Mouth: slight asymmetry |
| III (Moderate dysfunction) | Mild difference to gross inspection between sides Normal symmetry and tone at rest Forehead: slight to moderate movement Eye: compete closure, requires effort Mouth: slightly weak, requires effort |
| IV (Moderately severe dysfunction) | Obvious weakness to gross inspection between sides Normal symmetry and tone at rest Forehead: no motion even with effort Eye: incomplete closure even with effort Mouth: asymmetric with maximum effort |
| V (Severe dysfunction) | Only barely perceptible motion to gross inspection Asymmetry at rest Forehead: no motion even with effort Eye: incomplete closure even with effort Mouth: slight movement even with maximum effort |
| VI (Total paralysis) | No movement |
Evaluation should include pure-tone audiometry and electrophysiologic testing. A high-resolution CT of the temporal bone is the imaging study of choice for evaluating bone changes (from mastoiditis, temporal bone trauma, cholesteatoma, or neoplasm) whereas MRI with contrast is helpful when inflammation (eg, herpes zoster oticus) or neoplasm affecting the nerve is suspected. Laboratory studies should include a CBC with differential and ESR or CRP. If these are clinically suspected, autoimmune serologies and Lyme titers can be obtained.
If the facial nerve paralysis is caused by traumatic injury, management depends on the onset of paralysis; immediate and complete paralysis will often benefit from surgical decompression. In delayed or partial dysfunction, spontaneous recovery is likely and surgical intervention may not be beneficial. For idiopathic paralysis (Bell Palsy), initial medical therapy is targeted to reducing inflammation and targeting a possible viral etiology. A corticosteroid course should be initiated, either prednisone or prednisolone. Previous recommendations for the use of acyclovir (or similar antiherpetic analog) have recently been questioned. A large-scale randomized double blind study by Sullivan et al demonstrated no benefit for acyclovir, but significant benefit for prednisolone in the treatment of Bell Palsy. If the paralysis progresses, surgical decompression may be beneficial. Most experts also argue a significant benefit to performing surgical decompression of the facial nerve if a patient progresses to severe paralysis, provided the decompression is performed within 14 days of the onset of paralysis. Severe paralysis is usually defined as developing greater than 90% degeneration on electroneurography (ENoG) and lacking voluntary motor potentials on EMG.
The vast majority of cases of foreign bodies in the EAC occur in children, or mentally impaired adults. Many otologic foreign bodies, if carefully selected, are actually amenable to removal under direct visualization in an emergency department or primary care office setting. In a large series of over 600 cases of ear foreign bodies, Shulze et al found an overall 77% success rate for the removal under direct visualization by emergency physicians. It is important to note that the majority of those foreign bodies successfully removed in this manner fit the category of “soft, irregular” material such as paper or cotton, and “pliable or rubber-like” such as silly putty or erasers. Success with hard objects, and especially spherical objects such as plastic beads was markedly lower. Thus, an argument can be made for a single attempt by the pediatrician or emergency physician under direct visualization, if the object meets the former criteria. This should be tempered, however, with the understanding that complication rates are much higher with removal under direct vision. Complications of foreign body removal most commonly include canal wall lacerations (47%), with tympanic membrane perforations less common (4%). More serious complications such as ossicular chain injury and oval window perforation are possible but rare. When initial foreign body removal is performed instead by the otolaryngologist using binocular otomicroscopy either in the office or operative setting, the complication rate is quite low (6.3%).
Foreign body removal using binocular otomicroscopy is the primary method used by most otolaryngologists. Specific techniques depend on the characteristics of the foreign body. Objects with sharp edges can often be grasped with alligator or duck-bill forceps. Soft objects are often amenable to removal with otologic suction. Removal of spherical and hard, irregular objects requires more finesse. In these cases a 90-degree probe is invaluable. The probe is carefully guided behind the object under binocular otomicroscopic visualization. Then the probe is rotated along its axis to bring the end behind the object. The object is then guided out of the EAC. Another special case is an insect within the EAC, most commonly cockroaches. This can be quite alarming to the patient if the insect is still alive. The proximity of the insect to the tympanic membrane translates movement of the insect to distressingly loud perceived sound levels. In such cases the external ear canal can be gently irrigated with either mineral oil or lidocaine to suffocate the insect followed by prompt removal.
Some non-otolaryngologists advocate the use of gentle irrigation to attempt dislodgement of EAC foreign bodies. This technique can be used successfully, but requires caution. First, if there is any suspicion of tympanic membrane perforation, irrigation is contraindicated as it could flush debris into the middle ear space. Second, if the foreign body is composed of vegetable material (such as a popcorn kernel) irrigation should be avoided. If the object is not successfully flushed out, subsequent swelling of the vegetable foreign body can result in extreme pain as the EAC skin is compressed against the bony canal. Removal, once this occurs can be problematic, and may require general anesthesia and the use of an operating microscope. For this reason, otologic medications are also contraindicated in vegetable material otologic foreign bodies. The third condition where irrigation (and otologic medications) is specifically contraindicated is in the case of EAC button-batteries.
While the previously described foreign bodies can be managed on an outpatient basis, the finding of a button battery in the EAC is considered an emergent situation, requiring urgent removal by an otolaryngologist. If left in place for any length of time, batteries in the EAC can result in severe complications. In an early description of this problem by Kavanagh et al, 100% of patients experienced multiple, serious sequelae. These included tympanic membrane perforation or total destruction (75%), marked dermal destruction with bone exposure (88%), impairment of hearing (38%), ossicular chain erosion (25%), and even facial nerve paralysis (13%). Both leakage of corrosive battery acid and electrical current discharge resulting in chlorine gas and sodium hydroxide by electrolysis have been hypothesized to contribute to the destructive effects of button batteries. Removal is accomplished under binocular otomicroscopy in the operating room, and sometimes requires piecemeal removal of the battery. Following removal of the battery, the EAC should be flushed with copious amounts of saline, and careful inspection of the external canal and tympanic membrane should be performed.
Otitis externa is an infection of the EAC, usually from bacterial species such as Pseudomonas, Proteus, Klebsiella, Streptococcus, and Enterobacter. Evaluation and management of a patient with suspected otitis externa includes a complete history and physical examination with emphasis on the otologic examination. Patients will often give a history of recent water exposure to the external ear, such as from swimming, or other predisposing factors such as chronic hearing aid use. The external pinna should be manipulated gently. In otitis media this should not elicit pain, whereas for patients with otitis externa movement of the pinna is extremely painful. On handheld otoscopy the canal wall will appear edematous and erythematous. Sometimes the edema is severe enough the tympanic membrane cannot be visualized. In this case, insertion of a Pope otowick is indicated to carry ototopical medications the length of the external ear canal past the obstructed site. Severe cases may require frequent suction debridement under microscopic visualization. Patients should be placed on ototopical antibiotic drops containing a topical corticosteroid, such as ciprofloxacin 0.3%/dexamethasone 0.1% suspension (Ciprodex). Some (largely nonotolaryngologist) physicians allow the use of hydrocortisone 1%/polymyxin/neomycin (Cortisporin) as an alternative. This is not recommended for several reasons. First, several authors have demonstrated up to 10% risk of contact dermatitis with polymyxin/neomycin ototopical drops. Second, if a possibility of tympanic membrane perforation exists, these drops carry the possibility of ototoxicity, according to laboratory studies in animals; they are not approved for the middle ear use (unlike fluoroquinolones). Additionally, some studies have demonstrated faster pain relief with Ciprodex compared to Cortisporin. It is essential to note that failure to respond to appropriate therapy within 48-72 hours should prompt the clinician to reassess the patient to confirm a diagnosis of otitis externa.
Of note, patients with a history of diabetes (or any immunocompromising condition) should demand more aggressive therapy targeted toward Pseudomonas. This will usually involve an ototopical fluoroquinolone, ototopical corticosteroid, and oral or IV fluoroquinolone therapy. In the past, this subset of otitis externa carried the misnomer “malignant otitis externa” due to the high mortality rate even with surgical debridement and antibiotics. Modern antibiotic therapy and earlier diagnoses and intervention have dramatically improved outcomes, and mortality from this disease is now relatively rare.
Otomycosis is otitis externa due to a fungal infection, usually due to Aspergillus or Candida species. On otoscopy, there is usually far less edema and erythema than with bacterial infection. The use of ototopical antibiotic solutions will not improve these patients, and usually worsens their condition. Otomycosis can be notoriously difficult to treat, but many cases do respond to suction debridement followed by acidic eardrops and a topical corticosteroid. A commonly used topical preparation is acetic acid 2% with hydrocortisone 1% (Vosol HC). Topical antifungals such as nystatin or amphotericin B are also available but use of these should be reserved for difficult cases under the care of an otolaryngologist.
Classification of common middle ear pathology is often poorly understood by nonotolaryngologists. There are multiple disease processes of the middle ear which include the root term “otitis media.” Additionally, the acronyms used to represent diseases of abnormal middle ear fluid are quite similar.
The first condition, AOM, represents what is commonly referred to as “a middle ear infection.” The typical patient is a young child, with history of upper respiratory tract symptoms, fever, and pulling at one ear. Handheld otoscopy will reveal a bulging, erythematous tympanic membrane. Unlike otitis externa (above) there is no pain with manipulation of the auricle. Several studies from Europe have demonstrated that the majority of AOM cases resolve spontaneously without intervention. Nevertheless, in the United States most parents would be unhappy with a decision not to treat, and antibiotic therapy for AOM is routine. Usual pathogens include bacteria such as Streptococcus, Haemophilus, and Moraxella. The latter two are often resistant to penicillins and thus treatment with amoxicillin may not clear the infection; thus many practitioners recommend a second-generation cephalosporin. Failure to respond to these agents often necessitates a second-line antibiotic such as amoxicillin-clavulanic acid (Augmentin).
Otitis media with effusion (OME) is defined as fluid in the middle ear but no active signs of an infection. OME often results from eustachian tube dysfunction, which predisposes to accumulation of a sterile serous fluid in the middle ear cleft that does not clear. OME is common in young children, with a prevalence approaching 30% according to some reports. Patients with OME present with complaints of muffled or decreased hearing. Examination reveals fluid in the middle ear cleft. Chronic serous otitis media (CSOM) results when middle ear fluid after an episode of AOM fails to clear after a reasonable time span (4-6 weeks).
The most common surgical intervention to treat these problems is myringotomy and tympanostomy (M&T), so-called “ventilation tube” placement. Basic indications for M&T include multiple episodes of AOM (four episodes in 6 months, or six episodes in 12 months), CSOM with hearing impairment present for 3 months or longer, or presence of complications of AOM. Some authors have advocated for a more stratified approach to indications, where patients presenting with problems at an early age warrant surgical intervention with less stringent criteria.
Vestibular schwannomas (also sometimes referred to by the misnomer “acoustic neuromas”) represent a nonmalignant but neoplastic proliferation of Schwann cells ensheathing the eighth cranial nerve. These tumors represent nearly 10% of all intracranial tumors, and usually present with a unilateral high-frequency sensorineural hearing loss, followed later by development of imbalance symptoms. Even a mild degree of hearing loss may be misleading, as sensory processing (as evidenced by speech discrimination scores) are often more impaired than pure-tone averages would predict. Tinnitus and true vertigo are less common symptoms. Interestingly, these tumors arise more often from the vestibular division than the auditory division of cranial nerve eight. The symptoms associated with vestibular schwannomas are associated with compressive effects from the neoplastic growth. In the case of larger tumors, patients can sometimes present with facial nerve weakness. One important syndrome associated with vestibular schwannomas is neurofibromatosis-2 (NF2). Patients with NF2 can present with bilateral vestibular schwannomas, and consideration of hearing preservation strategies thus is extremely important for these patients.
Presumptive diagnosis of vestibular schwannoma is usually made by MRI with gadolinium. Schwannomas enhance brightly on T1 or T2 weighted images with gadolinium. Other diagnostic tests can include auditory brainstem response (ABR), electronystagmography (ENG). All patients with suspected vestibular schwannomas should have audiometric testing (pure-tone averages and speech discrimination scores) performed.
Management of vestibular schwannomas remains controversial. Many authors advocate that small schwannomas confined to the IAC can be successfully watched via serial imaging. Tumors demonstrating no growth (<2 mm) can continue to be watched, while most authors would argue for intervention if more than 2 mm growth occurs. If intervention is elected, treatment can be microsurgical (often involving combined neurosurgical and otologist collaboration) or by stereotactic radiosurgery (gamma knife) for small tumors. The goal of treatment is eradication of the tumor while preserving hearing and facial nerve function when possible. Preservation of facial nerve function (House-Brackman grade 2 or better) is generally successful in up to 70% of patients, regardless of surgical approach used.
Dizziness is an extremely common phenomenon, and has been estimated to affect as many as 30% of patients. An important distinction should be made at this point between various sensations commonly described by patients as “dizziness.” For patients describing dizziness symptoms, an attempt to elicit a more accurate description should always be sought. Vertigo is defined as the illusion of rotation. This should be distinguished from sensations of imbalance or unsteadiness, or of almost losing consciousness (presyncope).
Benign paroxysmal positional vertigo (BPPV) is the most common cause of acute-onset vertigo. Patients will describe sudden onset of intense vertigo lasting seconds rather than minutes, usually brought on by changes in head or body position relative to gravity. There is sometimes an associated history of head trauma. The etiology is thought to be due to dislocation of micro-crystals of calcium hydroxyapatite (otoconia) from the vestibule into the posterior semicircular canal. Certain head movements cause the otoconia to abnormally trigger copular deflection and thus elicit imbalanced vestibular input to the brainstem triggering intense vertigo. Diagnosis of BPPV can be made by positional testing, such as the Dix–Hallpike maneuver. In this test, the patient’s head is turned to one side and the patient is laid into a recumbent position with the head maintained in the rotated position. Elicitation of vertigo, often accompanied by the expected rotatory nystagmus, essentially confirms the diagnosis. Treatment consists of directed repositioning techniques such as the Epley maneuver. These maneuvers are designed to rotate the otoconia through the semicircular canal, depositing them back in a more physiologic location within the vestibule. Often several treatments are required, and patients can be instructed in the self-application of these maneuvers.
Méniére disease is characterized by waxing and waning sensorineural hearing loss (typically low frequency more than high), episodes of vertigo, sensation of aural fullness, and tinnitus. Hearing loss typically follows an episodic but slowly progressive course, with times of worse hearing followed by partial recovery. The hearing loss (and vestibular dysfunction) is most commonly unilateral, although bilateral disease can develop. The vertigo attacks associated with Méniére disease can be debilitating, and are often accompanied by nausea, vomiting, and inability to perform normal activities.
Diagnosis of Méniére disease is made based on clinical presence of the tetrad of symptoms, combined with evidence of hearing loss and vestibular dysfunction. The episodic nature of the disease is an important characteristic; a single episode of hearing loss and vertigo should not prompt a diagnosis of Méniére disease. In this case, viral labyrinthitis is a more likely culprit.
First described in 1861 by Prosper Méniére, the pathogenesis of Méniére disease remains essentially unknown. It is thought to relate to dilation of the membranous labyrinth, possibly from dysfunction within the endolymphatic sac. Anatomic cadaver studies have demonstrated endolymphatic hydrops (swelling of the scala media and endolymphatic sac) in patients with Méniére disease. Unfortunately, these anatomic changes have also been demonstrated in presumably normal (or at least asymptomatic) patients.
The mainstay of treatment for Méniére disease remains medical therapy. Patients are typically begun on a low-salt diet initially. Patients are also instructed to avoid caffeine, nicotine, and alcohol. Diuretics can be added, along with vestibular suppressants such as diazepam. Antihistamines (meclizine, dimenhydrinate, etc) have also demonstrated benefit in ameliorating vertigo symptoms associated with Méniére disease.
Patients suffering from severe, incapacitating vertigo and failing medical therapy can be offered several surgical interventions. Many authors advocate unilateral chemical ablation of the vestibular system. This is most often accomplished by transtympanic injection of gentamycin, and while efficacious for vertigo resolution is associated with sensorineural hearing loss in up to 25% of patients. For patients with intact hearing, many experts feel endolymphatic sac decompression and shunting can offer significant relief with preservation of hearing, although this remains unproven in randomized controlled trials. Similarly unproven, vestibular neurectomy (selective sectioning of the vestibular branch of the eighth cranial nerve) may conserve hearing and provide vertigo relief. For patients with severe hearing loss and vertigo, a total transmastoid labyrinthectomy relieves vertigo in over 90% of patients, at the cost of complete hearing loss on the affected side.
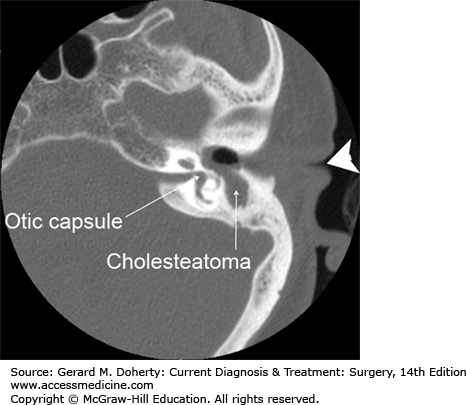
A cholesteatoma is a cyst-like, expansile lesion of the temporal bone consisting of stratified squamous epithelium and trapped desquamated keratin. It occurs in the pneumatized temporal bone, most commonly the middle ear and mastoid (Figure 15–2). There are two types of cholesteatoma (acquired and congenital) with the former being the most common. Acquired cholesteatomas arise from either retraction pockets within the tympanic membrane, or secondarily from a tympanic membrane perforation. Congenital cholesteatomas are thought to arise from epithelial cell rests that fail to undergo apoptosis during development. Whatever the origin, once formed cholesteatomas behave in a locally destructive manner. Bone erosion is common, especially of the ossicular chain but also potentially the bone surrounding the inner ear (the otic capsule). If left untreated cholesteatomas can even invade intracranially.
Figure 15–2.
Cholesteatoma of the left middle ear. Large arrowhead indicates the external ear canal. Note the soft tissue density (cholesteatoma) in the middle ear space and the absence of any visible ossicular chain. Part of the bony covering of the inner ear semicircular canal (the otic capsule) has also been eroded.
Early cholesteatomas have few if any symptoms, and usually start with a slowly progressive hearing loss. If an infection develops in a cholesteatoma, a foul otorrhea will develop, and this sometimes is the presenting symptom. If a cholesteatoma is suspected, careful inspection under binocular otomicroscopy is essential. All debris must be removed to allow a full visualization of the entire visible portion of the tympanic membrane. Cholesteatomas will appear as a whitish keratin mass. A pneumatic otoscopy test is essential; if vertigo is elicited the surgeon must suspect erosion into the inner ear structures.
The treatment for cholesteatoma is surgery, usually involving removal of the mastoid air cell septations by otologic drill, exposing the middle ear space. This accomplishes two goals—providing safe visualization and access and removal of all cholesteatoma tissue. This procedure is performed under careful microscopic visualization as many important structures (such as the facial nerve and the inner ear) should be preserved. The primary goal of surgery is creation of a safe, dry ear. All other considerations, including preservation of hearing, take second place.
DISORDERS OF THE NOSE AND PARANASAL SINUSES
The nose and paranasal sinuses serve to warm, filter, and humidify inspired air, to modulate vocalizations and speech, and provide for the sense of smell. The external nose consists of soft tissue and skin resting on a largely cartilaginous framework. The internal nose (nasal cavity) begins at the nasal vestibule anteriorly and extends posteriorly to the choana (which forms the boundary between the nasal cavity and the nasopharynx).
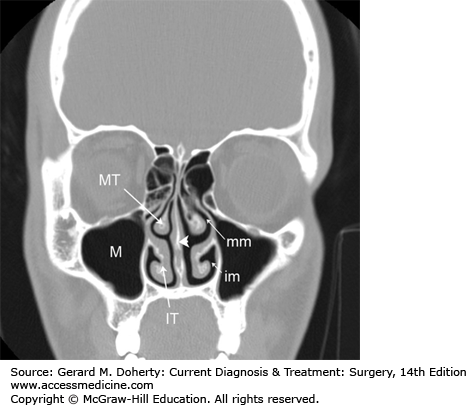
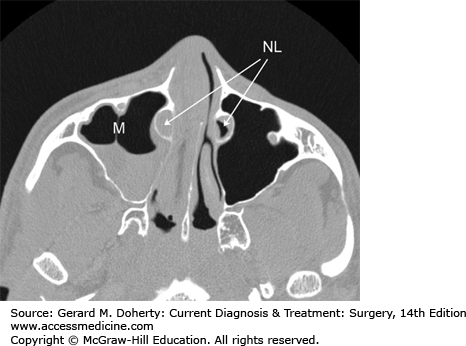
The nasal cavity (Figure 15–3) is divided in the sagittal plane in largely symmetric halves by the nasal septum. These cavities are partially filled by the three turbinates (superior, middle, and inferior, and occasionally a fourth supreme turbinate), which arise from the lateral nasal wall. The spaces below each turbinate are called meati (eg, superior meatus, middle meatus, and inferior meatus). These meati are important for localizing the outflow tracts of the various paranasal sinuses, which drain in a characteristic pattern. The nasolacrimal duct drains into the inferior meatus. The maxillary, frontal, and anterior ethmoid sinuses all drain into the middle meatus. The sphenoid sinus and posterior ethmoids drain into the superior meatus. Additionally, the olfactory nerve endings (the end organ for the sense of smell) are located on the superior nasal septum and superior turbinate mucosa.
The paranasal sinuses consist of hollow cavities that derive from pneumatization into the frontal, ethmoid, sphenoid, and maxillary bones of the craniofacial skeleton. They are lined with respiratory epithelium (pseudostratified ciliated columnar epithelium), which serves to circulate and drain mucous along with entrapped particulate matter. They are normally air-filled but can become fluid-filled if the ostia become obstructed by inflammation, anatomic problems, or disease process.
As is the case for foreign bodies of the external ear, nasal foreign bodies are typically encountered in children. Typical presentation is a young child with several day history of unilateral foul rhinorrhea. Oftentimes, the offending foreign body was inserted days to weeks before symptom onset, and the patient or parents may not recall a specific event precipitating the symptoms.
Offending foreign bodies can include vegetative, inert (plastic/metal) material and button batteries. Unlike external ear foreign bodies, nasal foreign bodies should always be treated as relative urgencies regardless of the type of object present. This is because of the anatomic relationship of the nose to the upper airway—if the object becomes dislodged, it could easily become an airway foreign body (a true emergency).
For this reason, many otolaryngologists advocate recommend removal of all but the most anteriorly placed nasal foreign bodies under general anesthesia using endoscopic visualization. The threshold for performing retrieval under sedation should likewise be low. In our practice, nasal foreign bodies confined to the nasal vestibule, or easily visible by anterior rhinoscopy can be safely removed without sedation in a cooperative patient. Young children, superior or posterior location or difficult visualization all result in removal in the operating room with endoscopic visualization. Another advantage to removing nasal foreign bodies in this manner is that postremoval inspection of the nasal cavity is easily performed. Oftentimes the nasal mucosa can be significantly inflamed and placement of absorbable material can prevent unwanted adhesions between opposing mucosal surfaces.
Nasal button batteries present a similar situation to external ear button batteries (see External Ear Foreign Body, above). The potential for extensive tissue damage from leakage of acid and electrical current discharge usually necessitates removal under general anesthesia. Following removal, extensive flushing with normal saline and careful inspection of the nasal cavity should be performed.
Invasive fungal sinusitis is almost always encountered in immunocompromised patients, most often patients undergoing chemotherapy for malignancy, or poorly controlled diabetics. It is caused by uncontrolled infiltrative growth of usually non-pathogenic fungal organisms. Offending agents are ubiquitous in the environment, and are commonly found in the nasal secretions of healthy normal patients. The two most common fungi are Aspergillus and Rhizopus species. The latter is more aggressive and is termed Mucormycosis. Rhizopus tends to preferentially grow in acidic environments, and is thus found more often in the setting of diabetic ketoacidosis. Even with early diagnosis and maximal surgical therapy, and modern antifungal agents, the disease still carries a significant mortality rate. This varies depending on causative agent, from approximately 10% (Aspergillus) to 30% (Rhizopus).
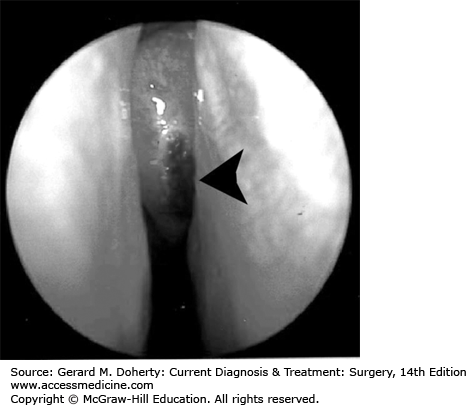
The index of suspicion for invasive fungal sinusitis must be high for any immunocompromised patient, as the symptoms can be subtle and the disease rapidly progressive. Patients are usually ill appearing and complain of facial pain, headache, nasal discharge, and may have mental status changes. Careful inspection of the face, oral cavity, and nasal cavity is mandatory. Dark ulcers (Figure 15–4) may be noted on the anterior face of the middle turbinate, inferior turbinate, lateral nasal wall, septum, or palate. Cranial nerve deficits may be noted in later stages of the disease.
If invasive fungal sinusitis is suspected, biopsies should be taken of the suspicious areas and sent for immediate pathologic examination. The pathologist should be alerted that invasive fungal sinusitis is suspected, so that special fungal stains can be performed. A worrisome finding is lack of bleeding at the biopsy site, as this may signify retrograde tissue infarction secondary to angioinvasive fungus.
Once invasive fungal sinusitis is confirmed, therapy consists of rapid reversal of immunosupression followed by aggressive surgical debridement of all necrotic tissue (down to healthy, bleeding tissue) and systemic antifungal therapy. Two commonly used agents are voriconazole and amphotericin B. Usually multiple surgical debridements are required. Typically, patients do not recover unless the underlying immunosuppression is resolved; for example absolute neutrophil counts greater than 500 or diabetic ketoacidosis rapidly reversed. Adjuvant therapy with donor neutrophil transfusion is an emerging treatment addition especially in cases failing to respond to conventional treatment. A multidisciplinary team including otolaryngology, hematology/oncology, and infectious disease specialists should closely follow these patients. Many patients develop metastatic fungal infections and can develop necrotic cavitation at distant sites. This often occurs as the patient’s immune system recovers, and can result in lethal pulmonary hemorrhage, strokes, and other serious systemic sequelae.
Invasive fungal sinusitis should not be confused with other forms of fungal disease of the paranasal sinuses. In allergic fungal sinusitis, chronic host immunologic/inflammatory response to noninvasive fungal elements results in tissue eosinophilia, nasal polyps (hyperplastic growth that obstructs normal drainage and airflow), and bony remodeling.
Epistaxis most commonly arises from the anterior portion of the nasal septum, referred to as Little’s area. In this region, blood vessels derived from both the internal and external carotid anastomose to form Kiesselbach plexus. This rich blood supply can result in quite dramatic amounts of bleeding which can be distressing for the patient. Fortunately, many of these episodes respond to simple external pressure (pinching the anterior external nose) for 10 minutes.
The etiology of epistaxis is primarily related to disruption of nasal mucosa, thus exposing small blood vessels that can rupture. In children this often relates to nose picking. In adults, the etiology often relates to turbulent nasal airflow, such as from a deviated nasal septum. Other predisposing factors include drying of the nasal mucosa, and hypertension. The latter is particularly important in the acute management of epistaxis; often the bleeding will not be controllable until the accompanying hypertension is dealt with.
Epistaxis not responding to conservative management may require intranasal tamponade. Several different packing devices, including petrolatum gauze, thrombin containing collagen products, sponges, and inflatable balloons can be used to perform anterior packing. Anterior bleeding from Little’s area can also sometimes be controlled with topical silver nitrate chemical cauterization, usually under endoscopic visualization.
Another common source of epistaxis is bleeding from branches of the anterior ethmoid or sphenopalatine artery. Epistaxis from these often requires posterior packing (such as with Foley catheter occlusion of the choanae and complete obliteration of the nasal airspace with gauze packing). Patients requiring posterior packing should be hospitalized and placed on pulse oximetry. Of note, nasal packing materials should be covered with topical antibiotics before placement, and all patients with intranasal packing should be placed on antistaphylococcal antibiotics to prevent possible toxic shock syndrome. Epistaxis recalcitrant to anterior and posterior packing can be managed by surgical ligation of the offending arterial supply (internal maxillary, sphenopalatine, and anterior ethmoid) or by arterial embolization by an interventional radiologist.
Chronic medical management of patients predisposed to epistaxis includes management of hypertension, and promoting moist nasal mucosa. Patients are commonly placed on two to three times daily application of nasal saline spray, and topical petroleum jelly or antibiotic ointment to the anterior septum. Patients with severe, recurrent epistaxis should be evaluated for possible systemic disease (such as hereditary hemorrhagic telangiectasia, Wegener granulomatosis, etc).
Rhinosinusitis refers to inflammation of the mucosal lining of the nose and paranasal sinuses. Acute rhinosinusitis is present for less than 3 weeks, and is usually precipitated by a viral upper respiratory tract infection. It is important to emphasize that only a minority of cases of acute rhinosinusitis become complicated by bacterial superinfection (0.5%-2%). Similarly, change in color of nasal discharge is not a specific sign of bacterial rhinosinusitis.
Symptoms initially reflect the precipitating upper respiratory viral infection (cough, sneezing, fever, nasal congestion, facial pain/pressure, rhinorrhea, and sore throat) followed by development of rhinosinusitis symptoms. A set of diagnostic symptoms for rhinosinusitis (both acute and chronic) have been established. Patients must have two major, or one major and two minor criteria. These criteria are outlined in Table 15–3.
| Major Criteria | Minor Criteria |
|---|---|
| Facial pain or pressure | Headache |
| Nasal obstruction | Fever (for chronic rhinosinusitis) |
| Nasal discharge or purulence | Halitosis |
| Purulence in nasal cavity | Fatigue |
| Anosmia or hyposmia | Dental pain |
| Fever (for acute rhinosinusitis) | Cough |
| Ear pain/pressure/fullness |
Acute bacterial rhinosinusitis should be suspected if symptoms persist after 10 days, or worsen within 10 days after an initial improvement. Physical examination may reveal purulent nasal secretions, nasal mucosal erythema, and tenderness overlying the sinuses. Nasal endoscopy (with middle meatal cultures) can be very useful, and visualization of purulent secretions emanating from the osteomeatal complex should increase suspicion for bacterial rhinosinusitis.
Treatment of acute rhinosinusitis is largely conservative in nature. Nasal saline lavage helps to eliminate excess mucous and inflammatory mediators, and restore mucocilliary clearance. Topical decongestants (such as oxymetazoline) can help reduce mucosal edema and restore sinus ostia drainage. Use should be restricted to 3 days, as tachyphylaxis and dependence can result from overuse of topical decongestants. Mucolytics such as guaifenesin can help thin mucous secretions, allowing easier mucocilliary transport. The only therapy tested and confirmed by placebo-controlled trials is intranasal topical steroids (such as mometasone or flunisolide). Topical steroids have been demonstrated to reduce time to symptom resolution, both for bacterial and nonbacterial acute rhinosinusitis. It is important to note that antihistamines have no proven benefit in acute rhinosinusitis, and may actually cause symptom exacerbation by drying mucous secretions. Antibiotics should be reserved for patients suspected of having acute bacterial rhinosinusitis. Current recommendations specify first-line antibiotic therapy should consist of amoxicillin. After 7 days, if a patient fails to improve clinically a broader-spectrum antibiotic such as a fluoroquinolone, trimethoprim/sulfamethoxazole, azithromycin, or amoxicillin/clavulanic acid can be tried. All of these have greater than 80% efficacy in clearing acute bacterial rhinosinusitis.
Patients experiencing multiple episodes of acute rhinosinusitis should be evaluated carefully for predisposing conditions. These may include anatomic obstruction (which may be relieved by septoplasty and/or functional endoscopic sinus surgery), underlying impairment of mucociliary clearance (such as from immotile cilia/Kartagener syndrome, or cystic fibrosis), or immune system dysfunction. Complications of acute bacterial rhinosinusitis can include orbital cellulitis and subperiosteal abscess formation, meningitis, and cavernous sinus thrombosis.
Chronic rhinosinusitis is extremely common, and affects between 2% and 15% of people in the United States. It is defined as the presence of rhinosinusitis symptoms for greater than 12 weeks (major criteria from Table 15–3) combined with evidence of inflammation. The latter can include findings of purulent mucous in the middle meatus or ethmoid region, nasal polyps or polypoid degeneration of the nasal mucosa. Radiographic findings (Figure 15–5) can also document inflammation, most commonly by CT. Findings can include diffuse mucosal thickening, chronic bony remodeling, and sinus opacification.
Management of chronic rhinosinusitis is primarily by topical and systemic medication. Most otolaryngologists place chronic rhinosinusitis patients on topical nasal steroids for at least 1 month. At this time if symptoms persist, a CT scan may be useful in demonstrating any anatomic abnormalities that may be amenable to surgical correction. Surgery is aimed at removing the obstruction to natural mucous flow from the paranasal sinuses, and most patients will continue to require medication after surgery to prevent return of symptoms.
The etiology of chronic rhinosinusitis is not fully elucidated. Most otolaryngologists feel that there are several disease processes currently described together under the generic heading chronic rhinosinusitis. Tissue eosinophilia may play an important role in differentiating these groups; current molecular evidence supports this distinction. Future research will undoubtedly change our understanding of these disease processes and how they are managed.
As mentioned above, extended use of topical nasal decongestants (such as oxymetazoline) or other vasoconstrictors (such as intranasal cocaine) can lead to tachyphylaxis and mucosal dependence. The resulting severe mucosal edema, hyperemia, and nasal obstruction are termed rhinitis medicamentosa. Patients will report daily topical vasoconstrictor/decongestant use, and absolute dependence on these medications for any appreciable nasal airflow. It is not unusual for patients suffering from rhinitis medicamentosa to carry their topical vasoconstrictor medication with them due to their frequent use. This is often a telltale sign of dependence. On physical examination the nasal mucosa will be thickened, erythematous and edematous, and lack appreciable decongestion on topical decongestant application.
Treatment requires complete cessation of the offending agent. Patients should be started on nasal saline lavage and nasal topical steroids. Oral decongestants and a course of oral corticosteroids may help hasten symptom resolution and increase patient compliance. Resolution usually takes 3-4 weeks, and may require much more time in the case of long-term vasoconstrictor use, or intranasal cocaine abuse.
Complications of untreated rhinitis medicamentosa include poor healing after nasal surgery, septal perforation, and formation of synechiae. For this reason, it is important to recognize and treat rhinitis medicamentosa preoperatively, before embarking on any nasal surgical treatment.
DISORDERS OF THE ORAL CAVITY AND PHARYNX
The oral cavity is bounded anteriorly by the vermilion border of the lips, and posteriorly by the anterior pillars of the palatine tonsils. The superior aspect of the oral cavity includes the hard and soft palate, and inferiorly includes the lingual mucosa and the anterior two-thirds of the tongue. This region of the tongue is bounded by the circumvallate papilla, which lie along the sulcus terminalis and separate the oral tongue from the base of tongue (part of the oropharynx).
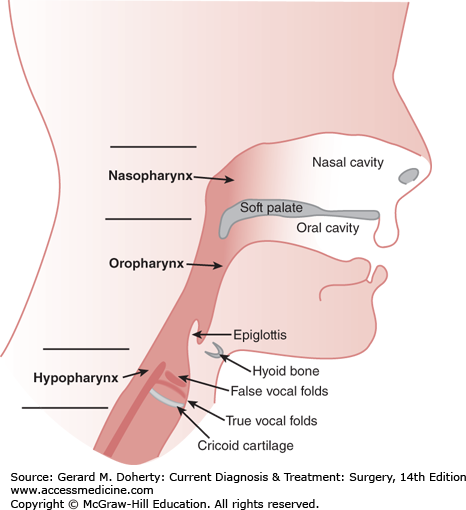
The pharynx connects the nasal and oral cavities to the esophagus and larynx. It consists of three segments—the nasopharynx, the oropharynx, and the hypopharynx (Figure 15–6). The nasopharynx begins as an extension from the posterior aspect of the nasal cavity and extends from the nasal choana to the soft palate. The oropharynx extends from the soft palate to the level of the hyoid bone, and is bounded laterally by the tonsillar pillars (the palatoglossal and palatopharyngeal arches). It includes the base of tongue, lateral/posterior pharyngeal wall, and tonsillar fossae. The hypopharynx extends from the level of the hyoid bone to the inferior aspect of the cricoid cartilage, and includes the pyriform sinuses, postcricoid region, and posterior hypopharyngeal wall.
Figure 15–6.
Relationship of the three sections of the pharynx. The nasopharynx extends from the nasal choanae to the soft palate. The oropharynx then extends from the soft palate to the level of the hyoid bone. The hypopharynx extends from the level of the hyoid bone to the level of the cricoid cartilage.
The primary functions of the oral cavity are related to chewing and swallowing (mastication and deglutition) and shaping of phonatory vibrations to produce intelligible speech. Taste buds on the dorsum of the tongue are responsible for basic taste perception—sweet, salty, bitter, and sour. This sensory information is transmitted to the facial nerve via the chorda tympani nerve for the anterior two-thirds of the tongue. General sensation of the tongue is carried by the lingual nerve. All sensory information from the posterior one-third of the tongue is carried by the glossopharyngeal nerve. Complex nuances of taste are mediated by olfactory receptors in the superior-most aspect of the nasal cavity and are not directly related to the tongue or oral cavity.
The tongue has four pairs of intrinsic muscles, which interdigitate throughout the tongue. These muscles act to lengthen or shorten the tongue, curl the apex and edges, and flatten or round the dorsal surface. The intrinsic tongue muscles originate and insert within the tongue itself. Extrinsic tongue muscles (genioglossus, hyoglossus, styloglossus, and palatoglossus) also act to protrude, depress, elevate, and retract the tongue. All motor function of the tongue is mediated by cranial nerve XII (the hypoglossal nerve).
The act of swallowing, or deglutition, is complex and consists of three main phases—oral, pharyngeal, and esophageal. The oral phase is under voluntary control, while the remaining phases proceed under reflex control. The oral phase of swallowing consists of preparation of the food bolus by mastication to soften and shape the bolus. Following this, oral transport ensues and the food bolus is transported to the posterior tongue. The anterior tongue then elevates against the hard palate, contracts and propels the bolus to the oropharynx. Simultaneously, the nasopharnyx is sealed off preventing nasal regurgitation. In the pharyngeal phase, several complex actions occur which elevate the larynx, temporarily halt respirations and protect the airway from aspiration, and relax the cricopharyngeus muscle to allow passage of the food bolus. The esophageal phase then propels the food bolus distally by means of sequential peristaltic contractions. Alteration of the timing or execution of any of these phases can result in dysphagia, or difficulty swallowing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree