http://evolve.elsevier.com/Edmunds/NP/
 Top 100 drug;
Top 100 drug;  Key drug. Alendronate is a key drug because it was the first in its class and is still commonly used.
Key drug. Alendronate is a key drug because it was the first in its class and is still commonly used.
Therapeutic Overview
The bony skeleton is made up of an outer shell of cortical dense bone that surrounds an internal honeycomb-like structure of trabecular bone. Bone is composed of a protein framework that hardens when the minerals calcium and phosphorus are deposited on it. Without enough calcium, bones get weak.
Bone is constantly changing. This occurs in an ongoing cyclic pattern that allows both proper bone health and appropriate use of calcium in the bones for other body functions, such as maintaining normal heart rhythm. Bone remodeling is a process in which old bone is removed and new bone is laid down. This process is often precipitated by microtrauma to the bones. The two stages of bone remodeling are resorption and formation. The first stage of remodeling is bone breakdown or resorption, when old bone is removed. The osteoclasts scoop away the bone over a period of about 2 weeks and are replaced by osteoblasts. The calcium in the broken-down bone tissue circulates throughout the body and is used for other key functions. The second stage, bone formation, follows when new bone fills in the spaces from which the old bone was removed. Bone resorption followed by bone formation is referred to as coupling. During childhood and through early adulthood, new bone is added to the skeleton faster than old bone is removed. As a result, bones become stronger, larger, and denser. Peak bone mass, the maximum amount of bone that a person can have, is reached at between 20 and 30 years of age. After age 30, bone is removed faster than it forms. In adults, approximately 25% of trabecular bone is resorbed and replaced every year, compared with only 3% of cortical bone.
Pathophysiology
Osteoporosis is a common pathologic process of the skeleton in which bone strength is decreased. This predisposes the individual to increased risk of fracture. Bone strength is achieved through both bone density and factors such as bone architecture, cellular turnover, mineralization, and microfractures and other damage that all affect the quality of the bone. In osteoporosis, the normal coupling mechanism of bone breakdown and bone regrowth appears not to keep up with the constant microtrauma to trabecular bone, and either too little bone is formed or too much bone is removed. This ultimately results in a loss in bone amount and strength. Osteoporosis also may be produced by long-term glucocorticoid use, including use of inhaled steroids for treatment of chronic obstructive lung disease. Box 39-1 lists risk factors associated with osteoporosis. In periods of rapid remodeling (e.g., after menopause), bone may be at an increased risk for fracture because newly produced bone is less densely mineralized, the resorption sites are temporarily unfilled, and maturation and isomerization of collagen are impaired.
When optimal bone strength is not attained by the time a person is in her thirties, this contributes to osteoporosis. Good nutrition and physical activity help with bone strength and so are important to individuals during growth and development. Accelerated bone loss can occur in postmenopausal women and elderly men and women, and it also can occur secondary to various disease states and medications. Studies have shown that bone loss in women accelerates rapidly in the first years after menopause. The lack of gonadal hormones is thought to increase the activity of osteoclast progenitor cells in both men and women. While these factors are all important in contributing to osteoporosis, overall, hereditary factors play the principal role in determining an individual’s peak bone strength and may account for up to 80% of the variance in peak bone mass between individuals. In contrast to postmenopausal bone loss, the bone loss that accompanies aging is associated with a progressive decline in the supply of osteoblasts in proportion to the demand. Bone homeostasis is maintained by calcium, vitamin D, and parathyroid hormone (PTH). Insufficient dietary calcium or impaired intestinal absorption of calcium due to aging or disease can lead to secondary hyperparathyroidism.
Disease Process
Osteoporosis now is widely recognized as a progressive systemic disease, characterized by low bone density (osteopenia) and microarchitectural deterioration of bone that predisposes patients to increased bone fragility and fracture. Because of decreased bone mass, fragility fractures result from trauma that would not cause normal bone to fracture. Osteoporosis of the spine may lead to crowding of internal organs, spinal cord compression, GI disorders, or restrictive lung disease. Increased mortality and morbidity are associated with limited physical activity, back pain, skeletal deformity, height loss, and kyphosis. Clinically significant osteoporosis of the spine is associated with significant morbidity, reduced quality of life, and soaring medical costs.
Women are more susceptible to osteoporosis because their peak bone mass tends to be 10% to 30% less than that of males. The prevalence of osteoporosis in men increases after age 80. Men have a shorter life span than women, so they account for only 21% of hip fractures. By the age of 90, only 17% of men have had a hip fracture, compared with 32% of women. Osteoporotic fractures present as clinical significant derangements in bone. They result from both low-energy trauma, such as falls, or sitting or standing in one position, and from high-energy trauma, such as a motor vehicle accident. Fragility fractures, which occur secondary to low-energy trauma, are characteristic of osteoporosis and may be the presenting problem to the clinician.
Assessment
Bone loss does not show up on conventional radiographic films until one fourth or more of the bone’s mineral content is gone, so bone mineral density (BMD) may not always be a good marker of clinical problems. The most commonly used technique for measuring bone loss is central dual energy x-ray absorptiometry (DXA), which measures the spine, hip, or total body. It is the most sensitive test and provides the most precise T-scores. Peripheral dual energy x-ray absorptiometry (pDXA) measures the wrist, heel, or finger. Quantitative computed tomography (QCT) measures the spine, hip, or total body. Ultrasonometry uses sound waves to measure density at the heel, shinbone, and kneecap. This technique can be performed easily in the office setting but is currently less sensitive than DXA.
The U.S. Preventive Services Task Force maintains that bone density testing is indicated for women and men with an increased risk for osteoporosis and routine screening to include all women 65 and older. If a woman is at least 5 years postmenopausal or has several risk factors, she should be strongly encouraged to have bone density testing and should be placed on therapy if the testing is indicative of osteoporosis or osteopenia. Box 39-2 lists indications for BMD testing.
A T-score represents the number of SDs above or below the mean BMD for the young, healthy female population (i.e., women who are younger than 35 years of age). A T-score of −1 signifies a 10% to 12% loss of bone mass, compared with mean values for young, normal adults. According to recommendations of the WHO Task Force for Osteoporosis, osteoporosis is defined as a T-score ≤2.5 in women without a history of fragility fractures (Table 39-2). Treatment generally is indicated if the patient is two or more standard deviations below the normal premenopausal level.
TABLE 39-2
WHO Criteria for Diagnosis of Osteoporosis for Postmenopausal Women and Men Aged 50 Years or Older
| T-Score∗ | Classification |
| ≥–1 | Normal |
| ≤ –1 to –2.5 | Osteopenia (low bone mass) |
| ≤ –2.5 | Osteoporosis |
| ≤ –2.5 + fracture | Severe osteoporosis |
∗T-score indicates the number of standard deviations below the average peak bone mass in young adult females.
In addition, researchers recently have developed a mathematical formula for predicting women’s risk of osteoporotic fracture. A woman’s risk for bone fracture, known as her “FRISK (fracture risk) score,” is obtained by using her BMD at the spine and femoral neck, the number of previous fractures sustained, her body weight, and a falls score derived from the number of falls in the past year. On a scale of 0 to 10, a FRISK score of 5.4 or higher is associated with an expectation of fracture. For every one unit increase in a patient’s FRISK score, the odds of having a fracture increase by 1.75. In initial trials, the formula has been shown to be as much as 75% accurate in predicting fracture in women with osteoporosis.
The longitudinal Study of Osteoporotic Fractures data suggest that women who have normal bone mineral density levels or mild osteopenia do not need a second BMD test again for at least 15 years. The long-term prognosis for osteoporosis is good if bone loss is detected in the early phases and proper intervention is undertaken to decrease the risk of falls. Patients can increase BMD and decrease fracture risk with the appropriate antiosteoporotic medication. These medications appear to have a limited effect on long-term osteoporosis because the body seems to develop a tolerance to the medications over time. Medication use must be undertaken with concomitant physical activity to increase muscle and bone mass, strength, and flexibility, and steps should be taken to reduce hazards such as loose rugs, defective stairs, poor lighting, and obstacles in the environment that present fall hazards.
Mechanism of Action
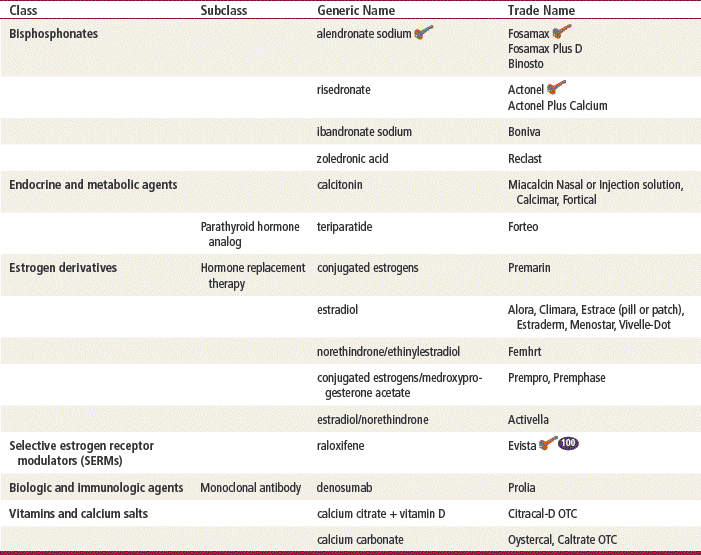
Bisphosphonates are nonhormonal agents that have an extremely high affinity for bone. Alendronate was the first of the newer bisphosphonates to be approved by the FDA for the treatment and prevention of osteoporosis. Risedronate is the second bisphosphonate to be approved for the treatment of osteoporosis. Ibandronate is the newest bisphosphonate and may be taken on a monthly basis. Bisphosphonates inhibit the activity of osteoclasts to normalize the rate of bone turnover. This results in an indirect increase in bone mineral density. The bisphosphonates are very specific to the skeleton; they reduce risk for both vertebral and nonvertebral fractures without demonstrating other benefits outside of the skeleton. One exception to this is ibandronate, which has not been shown to significantly reduce the incidence of hip fracture. These agents are incorporated into bone matrix but are not pharmacologically active thereafter. It is unknown whether the incorporated bisphosphonates, when released by resorption, could eventually interfere with bone remodeling.
A recent study of women who discontinued alendronate after 5 years showed a moderate decline in BMD and a gradual rise in biochemical markers but no higher fracture risk other than for clinical vertebral fractures compared with those who continued alendronate. BMD is half the equation; the nature of bone architecture is the other half. Therefore, these women may just have good bone architecture. These results suggest that for many women, discontinuation of alendronate for up to 5 years does not appear to significantly increase fracture risk. However, women at very high risk of clinical vertebral fracture may benefit by continuing treatment beyond 5 years.
SERMs
Another class of agents used in treating patients with osteoporosis is SERMs. Raloxifene and tamoxifen are both SERMs; however, raloxifene is the only one that is used for osteoporosis. Raloxifene was designed to exert an estrogenic effect in bone but not in reproductive tissue. It reduces resorption of bone and decreases overall bone turnover. Although raloxifene reduces the risk for developing vertebral fracture and increases hip and spine BMD, it has no significant effects on most nonvertebral fractures, including hip fracture. Raloxifene prevents endometrial thickening, reducing the risk of endometrial cancer, but it does not relieve the vasomotor symptoms of menopause. It also may reduce the risk of cardiovascular effects by lowering low-density lipoprotein cholesterol. It seems to exert antiestrogenic effects on breast tissue, which theoretically might decrease the risk of breast cancer. Raloxifene does, however, increase the risk of venous thromboembolism, similar to traditional estrogen therapy.
Hormones
Calcitonin is a naturally occurring hormone that is produced by C-cells in the thyroid gland. Although its mechanism of action in osteoporosis has not been fully delineated, calcitonin is known to block bone resorption through its potent inhibitory effects on osteoclasts. Calcitonin is a protein and therefore cannot be taken orally because it would be digested before it could work. Intranasal salmon calcitonin is 50- to 100-fold more potent than human calcitonin.
Calcitonin lowers serum calcium concentration primarily by direct inhibition of bone resorption. Osteoclasts are reduced in number and function, and osteocytic resorption is decreased. Calcitonin also has a direct effect on the kidneys. Through inhibition of tubular reabsorption, calcium, phosphate, and sodium are increased. However, urinary calcium is decreased rather than increased in some patients because calcitonin-induced inhibition of bone resorption has a greater effect on calcium excretion than does the drug’s direct renal action. In the Prevent Reoccurrence Of Osteoporosis Fractures (PROOF) study, treatment with nasal calcitonin produced a 1.2% increase in spinal BMD, along with a 36% relative risk reduction for new vertebral fractures. Calcitonin also has an analgesic effect through mechanisms that may involve increased release of endorphins. It may therefore produce beneficial pain relief after a vertebral fracture.
Synthetic human PTH, teriparatide, was approved by the FDA in 2003 and is the first approved agent for the treatment of osteoporosis that stimulates new bone formation. Once-daily injections stimulate new bone formation on trabecular and cortical bone surfaces through preferential stimulation of osteoblastic activity over osteoclastic activity. This effect is manifested as an increase in skeletal mass and an increase in bone turnover markers. By increasing new bone formation, teriparatide improves bone mass and bone strength. PTH also acts on the kidneys by reducing renal clearance of calcium. Patients treated with PTH along with calcium and vitamin D supplementation have statistically significant increases in bone mineral density (10% to 20% after 1½ years) at the spine and hip when compared with patients taking only calcium and vitamin D.
Treatment Principles
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


