5 OSTEOGENESIS
Bone formation (osteogenesis or ossification)
Bone develops by replacement of a preexisting connective tissue. The two processes of bone formation or osteogenesis observed in the embryo are: (1) intramembranous bone formation, in which bone tissue is laid down directly in primitive connective tissue or mesenchyme (Figures 5-1 and 5-2), and (2) endochondral bone formation, in which bone tissue replaces a preexisting hyaline cartilage, the template or anlage of the future bone (Figures 5-3 to 5-5).
Intramembranous bone formation
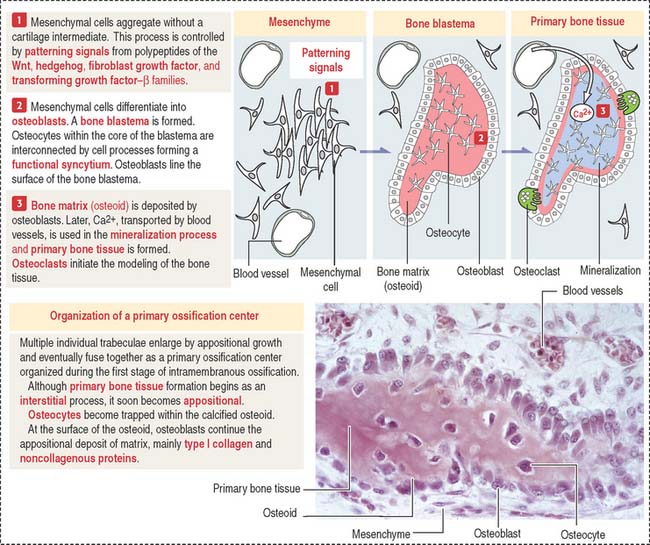
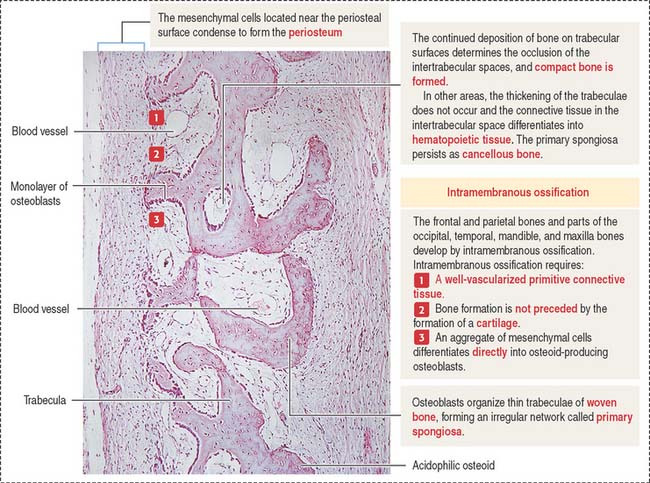
Membrane bones such as the flat bones of the skull develop by intramembranous ossification. Intramembranous ossification occurs in the following sequence (see Figure 5-1):
Box 5-A From osteoblasts to osteocytes
The final developmental events include:
Endochondral ossification
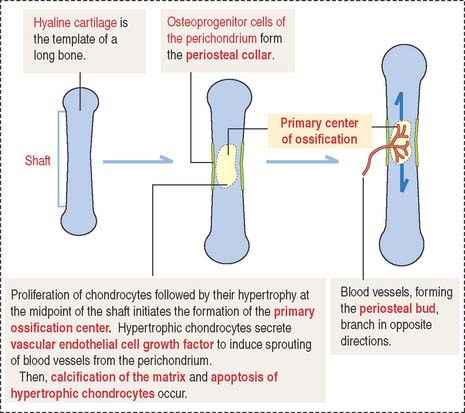
As in intramembranous ossification, a primary ossification center is formed during endochondral ossification (Figure 5-3). Unlike intramembranous ossification, this center of ossification derives from proliferated chondrocytes that have deposited an extracellular matrix containing type II collagen.
At the same time, the inner perichondrial cells exhibit their osteogenic potential, and a thin periosteal collar of bone is formed around the midpoint of the shaft, the diaphysis. Consequently, the primary ossification center ends up located inside a cylinder of bone. The periosteal collar formed under the periosteum by intramembranous ossification consists of woven bone. As we will discuss later on, the periosteal collar is converted into compact bone.
The following sequence of events defines the next steps of endochondral ossification (Figure 5-4):
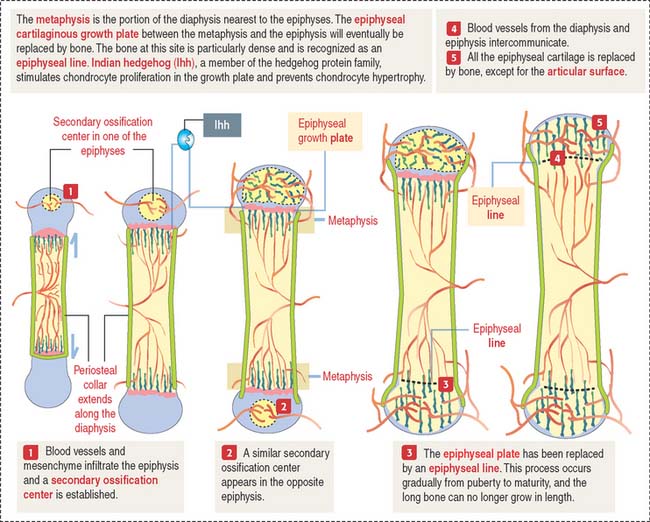
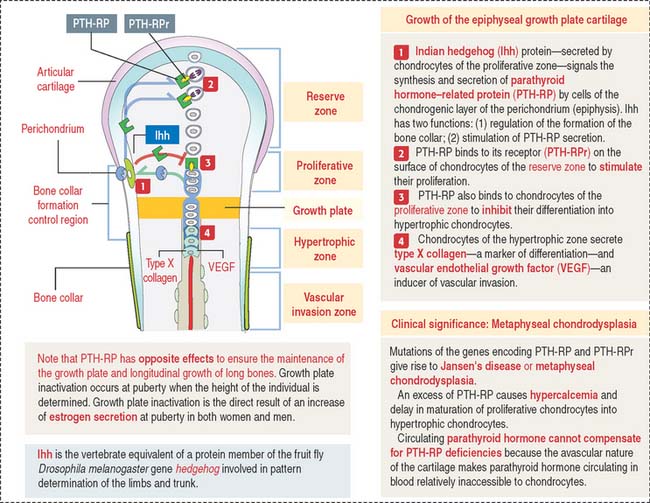
Secondary centers of ossification and the epiphyseal growth plate
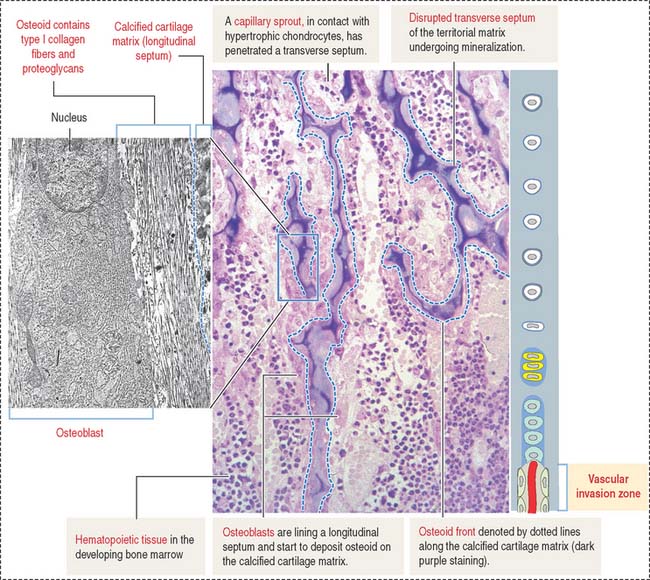
After birth, secondary centers of ossification develop in the epiphyses (see Figure 5-4). As in the diaphysis, the space occupied by hypertrophic chondrocytes is invaded by blood vessels and osteoprogenitor cells from the perichondrium. Most of the hyaline cartilage of the epiphyses is replaced by the spongy bone, except for the articular cartilage and a thin disk, the epiphyseal growth plate, located between the epiphyses and the diaphysis. The epiphyseal growth plate is responsible for subsequent growth in length of the bone.
Clinical significance: The epiphyseal growth plate and dwarfism
Indian hedgehog (Ihh), a member of the hedgehog family of proteins secreted by chondrocytes, regulates chondrocyte proliferation of the growth plate in a paracrine fashion and delays chondrocyte hypertrophy (see Figure 5-9). Ihh also regulates bone formation in the perichondrial collar. A lack of expression of Ihh protein in mutant mice results in dwarfism and lack of endochondral ossification. Essentially, Ihh maintains the pool of proliferating chondrocytes in the growth plate by delaying their hypertrophy. In addition, Ihh stimulates the expression of parathyroid hormone-related peptide (PTH-RP) in perichondrial chondrocytes adjacent to the articular surface. A feedback loop between Ihh and PTH-RP regulates the balance between proliferating and hypertrophic chondrocytes.
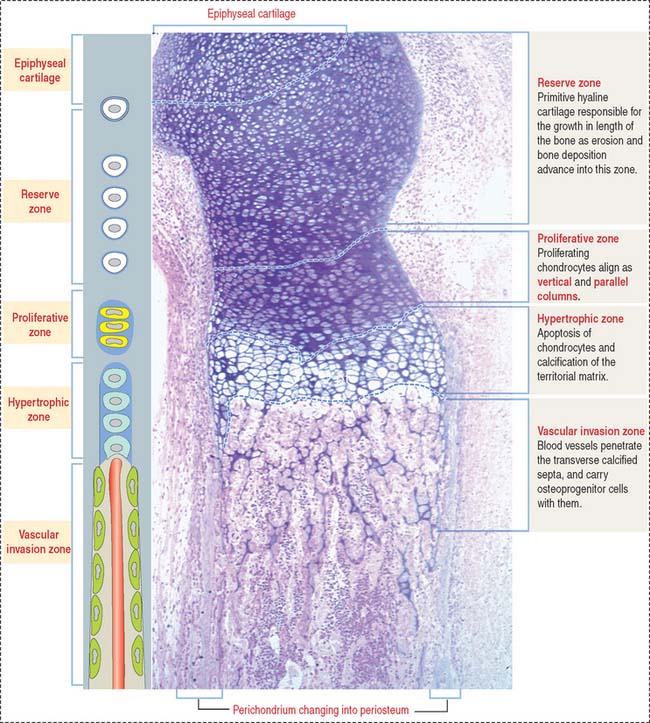
Zones of endochondral ossification
As we have seen, the deposition of bone in the center of the diaphysis is preceded by an erosion process in the hyaline cartilage template (see Figure 5-4). This center of erosion, defined as the primary ossification center, extends in both directions of the template, in parallel with the formation of a bony collar.
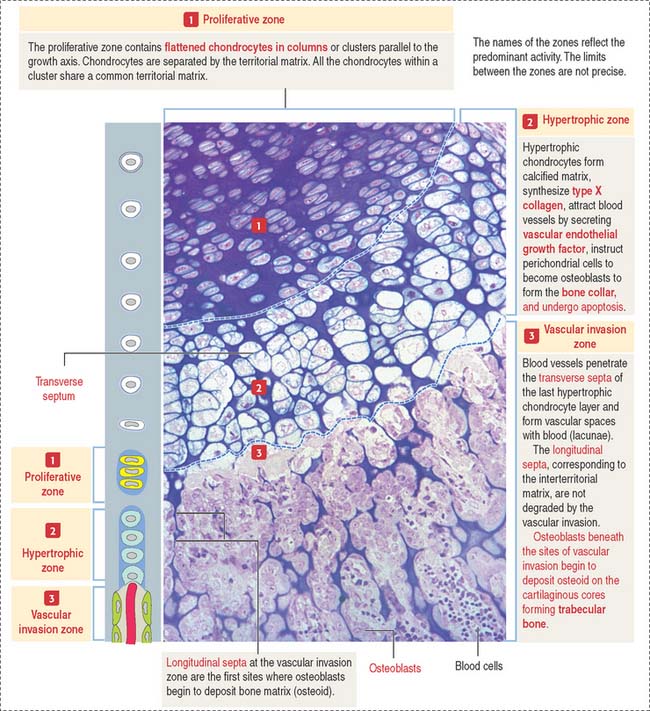
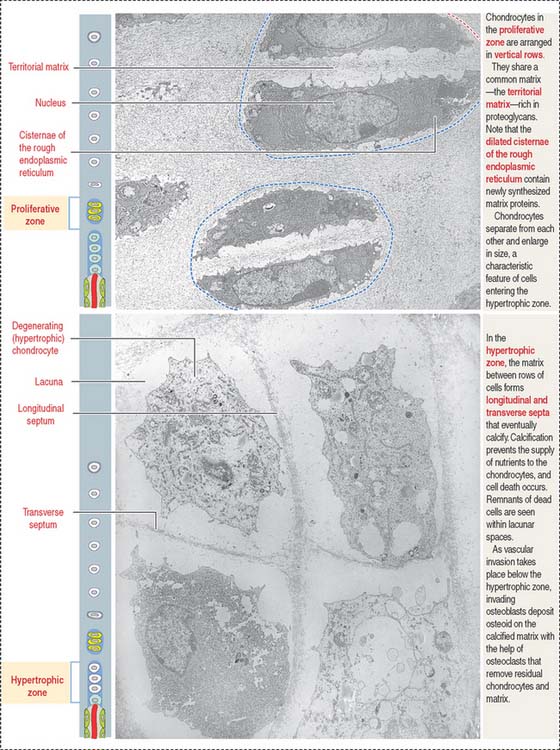
The continuing process of cartilage erosion and bone deposition can be visualized histologically (Figure 5-5). Four major zones can be distinguished, starting at the end of the cartilage and approaching the zone of erosion: