Chapter 29 Osteoarthritis
Introduction
Although diseases with the greatest consequent mortality (e.g. CVD, cancer) attract much of the public’s attention, musculoskeletal or rheumatic diseases are the major cause of morbidity throughout the world, having a substantial influence on health and quality of life, but not mortality, and inflicting a vast burden of cost on health systems. Musculoskeletal disease is a major cause of disability and handicap, and arthritis is the most prevalent form of musculoskeletal disease.1 Rheumatic diseases include more than 150 different conditions and syndromes with the common denominators being pain and inflammation. Five account for 90% of the cases — osteoarthritis (OA), rheumatoid arthritis (RA), fibromyalgia, systemic lupus erythematosus (SLE) and gout.1–4
Arthritis is a chronic disease affecting an estimated 43 million (20.8%) US adults and is the leading cause of disability in that country3 with OA reported to be the most common joint disorder in the world.4 In Western populations it is one of the most frequent causes of pain, loss of function and disability in adults. Radiographic evidence of OA occurs in the majority of people by 65 years of age and in about 80% of those aged over 75 years. In Australia in 2004, there were 3.4 million people (17% of the population) suffering from some form of arthritis, with 60% of these being females. Of this total, 1.39 million had OA and more than 438 000 had RA.5 Table 29.1 summarises the symptoms of OA.
Table 29.1 Symptoms of osteoarthritis
Integrative management of osteoarthritis
The early 1990s saw an upsurge of complementary and alternative medicine (CAM) use, based on reports that recognised the extensive use of treatments outside the realm of conventional medicine.6, 7 A recent review reported that patients with musculoskeletal conditions often employ CAM modalities8 in one form or another. Collectively the evidence demonstrates that some CAM modalities show significant promise, as for example herbal medicines, nutritional supplements, acupuncture, and mind–body medicine.
International expert panels have developed useful patient-focused, evidence-based integrative consensus recommendations for the management of hip and knee osteoarthritis.9, 10
These recommendations include those listed below.
Environment
Despite popular belief arthritic pain has no seasonal variation and is not more common in winter according to a study of 1424 patients with osteoarthritis, rheumatoid arthritis or fibromyalgia followed up over 24 years.11
However, associated atmospheric change and cold temperatures did impact on pain severity according to a study of 200 patients with OA. The authors postulated that the changes in cold temperature may contribute to changes in viscosity of synovial fluid which indirectly affects inflammation.12
Mind–body medicine
The non-pharmacological management of OA has been recently reviewed.13
Multi-modal cognitive behavioural and/or mind–body therapies, in combination with educational and information components (such as patient education and/or self-management programs) may be appropriate adjunctive treatments in the management of OA.14, 15 Cognitive behavioural therapy (CBT) has a positive effect on pain and other patient relevant outcomes in chronic pain management due to OA.16, 17 The best effect of CBT has been documented when it was employed together with physical activity as part of a multi-modal treatment program.18 Recent reviews have reported that there is limited evidence available for the role of hypnosis in the management of pain due to OA.14, 15
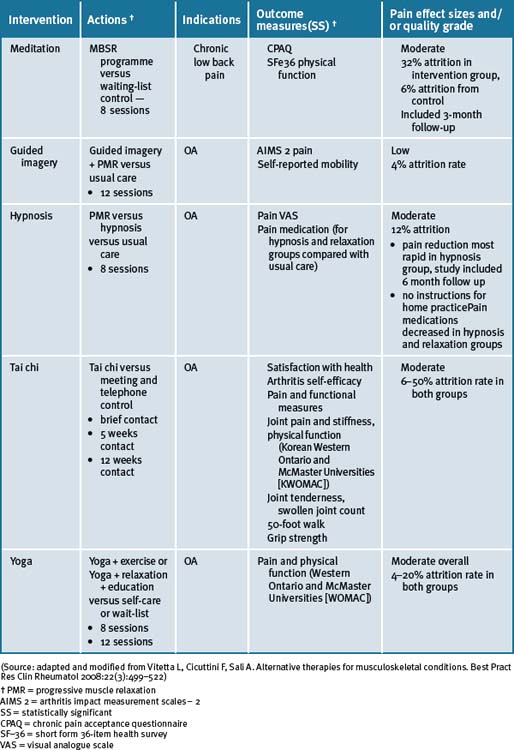
Mind–body medicine modalities are summarised in Table 29.2.
Physical activity/exercise
In primary care, physical activity is one of the foundations in the management of chronic musculoskeletal pain. Multiple research data supports the benefits of physical activity for alleviation of symptoms of OA; even vigorous exercise.19 A Cochrane review concluded there were no significant differences in benefit between high intensity and low intensity aerobic exercise on participants with OA of the knee for functional status, gait, pain and aerobic capacity.19 Both levels of exercise were beneficial. According to the review of non-pharmacological therapies for OA, exercise (aerobic, range-of-motion and strengthening) should be the leading intervention for OA patients, especially as obesity and being overweight are major risk factors for OA.19 However, recently another Cochrane review concluded that aquatic exercise appeared to have some beneficial short-term effects for patients with hip and/or knee OA while no long-term effects had been reported.20 Based on this data, it is possible to consider using aquatic exercise as the first part of a longer exercise program for OA patients.21
Both function and pain benefit from aerobic or strengthening activity for knee OA. This was confirmed in an RCT with older participants.22 Older disabled persons with OA of the knee had modest improvements in measures of disability, physical performance, and pain from participating in either an aerobic or a resistance exercise program. These data suggest that exercise should be prescribed as part of the treatment for knee OA.
In a recent 18 month clinical study investigating additive effects of glucosamine or risedronate for the treatment of osteoarthritis of the knee combined with home exercise,23 it was reported that there was improvement after 18 months in all groups using individual scales for evaluation of pain and function of the knee, however, with no significant differences observed between the groups. There were no additive effects of glucosamine or risedronate on the exercise therapy.23
Of interest, a large-scale study of 1279 middle-aged to elderly persons without knee OA (mean age 53.2 years; many of whom were classified as obese) who underwent both baseline and follow-up examinations after 9 years found that neither recreational walking, jogging, frequently working up a sweat, nor high activity levels relative to peers or even weight factors (Body Mass Index [BMI] above the median [7.7 kg/m2 for men and 25.7 kg/m2 for women]; mean BMI >30 kg/m2 for both) did not protect against nor increased the risk of developing knee OA.24
Tai chi and yoga
A recent systematic review has reported that there was some encouraging evidence suggesting that tai chi may be effective for pain control in patients with knee OA.25 However, the evidence was not persuasive for pain reduction or improvement of physical function. A prospective randomised control clinical trial of tai chi over a 12-week period demonstrated significant improvements in self-efficacy for arthritis symptoms (other than pain and function), total self-efficacy, tension levels and general health status, while pain and some lower limb functional scores showed moderate but not clinically significant improvements among older adults.26
A number of small studies have demonstrated that tai chi was effective for a number of people in different environments for the management of pain due to OA.27–30 In 1 study these findings supported the practice of tai chi as beneficial for gait kinematics in elderly people with knee OA.27 A longer-term application was suggested in order to substantiate the effect of tai chi as an alternative exercise in management of knee OA. A further study concluded that access to either hydrotherapy or tai chi classes could provide large and sustained improvements in physical function for many older, sedentary individuals with chronic hip or knee OA.28 In a 12-week study of the efficacy of tai chi to improve physical function, the study results demonstrated that the tai chi group reported lower overall pain and better WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index gauge physical function than the attention control group at weeks 9 and 12.29 All improvements disappeared after tai chi training ceased. In a study with older women with OA who were able to safely perform the 12 forms of sun-style tai chi exercise for 12 weeks, it was reported effective in improving arthritic symptoms, balance, and physical functioning.30 A longitudinal study with a larger sample size was suggested as necessary to confirm the potential use of tai chi exercise in arthritis management.
In a case series study investigating yoga and strengthening exercises for people living with OA of the knee, the study found functional changes and improvement in quality of life in traditional exercise and a yoga based approach.31 A single small study of the use of yoga for OA of the hands demonstrated improvements in pain and finger range of motion as compared to a control group.32
Nutritional influences
Diets
Obesity is a well-recognised risk factor for increased risk of OA, particularly of the knee and hip.33 A recent study showed that a high BMI was associated with progression of loss of joint-space width associated with knee OA but not hip OA.34 Dietary advice and intervention clearly have a place in musculoskeletal diseases and allow patients to experience some control over their own disease. Although there is no evidence for efficacy of trendy diets, a significant proportion of patients diagnosed with OA can benefit from excluding foods individually identified during the reintroduction phase of an elimination diet.35 Also a proportion of patients who follow a vegetarian or Mediterranean-type diet will experience a significant benefit.
Extra-virgin olive oil contains oleocanthol which acts as a natural anti-inflammatory, and may be of benefit for arthritic joint pains.36
Obesity and high BMI are also associated with more severe disease in terms of the amount of pain experienced37 and the need for joint replacement.38, 39
Dietary nutritional habits are an unavoidable widespread exposure for people for the development of chronic diseases that include musculoskeletal problems.39, 40 The Mediterranean diet reflects the dietary patterns characteristic of several countries in the Mediterranean basin during the 1960s. Typically, the diet comprises abundant plant foods that includes fruits, vegetables, wholegrain cereals, beans, nuts and seeds; minimally processed, seasonally fresh and locally grown foods; fish and poultry; olive oil as the main source of lipid, with dairy products, red meat and wine in low to moderate amounts.35, 40 Lifestyle changes that include adopting daily physical activity and smoking cessation together with changes in nutritional/dietary manipulations may lead to positive effects on musculoskeletal physiology with a consequent beneficial impact on the population health.
Early epidemiological studies have reported that diets rich in vitamins C and D slow progression of OA.41
Nutritional supplements
General
Dietary supplements, commonly referred to as natural medicine/compounds, and herbal medicines account for approximately $20 billion of annual sales in the US alone.8
A large number of dietary supplements are promoted to patients with osteoarthritis and as many as one-third of those patients have used a supplement to treat their condition.8 These sales for complementary medicine products indicate that glucosamine-containing supplements are among the most commonly used products for osteoarthritis.8 Glucosamine is available in multiple forms; the most common being glucosamine hydrochloride and glucosamine sulfate. Some marketed products contain a blend of these and many combine one of the forms with a variety of other ingredients.
Vitamins
Vitamin C and β-carotene
Perhaps the best study of vitamin C was the Framingham OA Cohort Study which demonstrated vitamin C intake was associated with reduced progression of arthritis in OA patients and lower incidence of knee pain.42 Specifically, a significant threefold reduction in risk of OA progression was reported, which related predominantly to a reduced risk of cartilage loss. This was documented for both the middle and the highest tertile of vitamin C intake. Those with high ascorbate intake also had a significantly reduced risk of developing knee pain. A significant, though less consistent, reduction in risk of OA progression was also seen for beta-carotene.42 Recently it has been reported that there was a beneficial effect with vitamin C intake on the reduction in bone size and the number of bone marrow lesions, both of which are important in the pathogenesis of knee OA.43, 44
Niacinamide
Small early trials have reported individual benefits with other vitamins. Namely, a double-blind control trial study of 72 OA patients treated with 3000mg/day in 6 divided doses of niacinamide over 12 weeks demonstrated improved pain scores, reduced global impact of OA, improved flexibility and reduced inflammatory markers (ESR) when compared to controls. Furthermore, the use of NSAIDs was reduced with use of niacinamide.45
Pantothenic acid
A small trial of 26 patients supplemented with 12.5mg/day pantothenic acid reported relief in severity of OA. However 3 patients reported the onset of general anaesthesia and leg weakness with the dosage, for which 12.5mg/day of pyridoxine was administered to elicit recovery.46
Folic acid and vitamin B12 cyanocobalamin
Folic acid (6400mcg) and cyanocobalamin (20mcg) consumed orally over a 2-month period demonstrated greater improvements in hand grip and number of tender joints when applied to 26 patients with OA of the hands when compared to NSAIDs.47 All of these studies require follow-up with properly designed trials.
Antioxidants
A recent systematic review concluded that there is presently no convincing evidence that antioxidants such as selenium, vitamin A, vitamin C or the combination product selenium ACE is effective in the treatment of any type of arthritis.48
Vitamin D
Vitamin D emerges as a vitamin that could stimulate the synthesis of proteoglycan by articular chondrocytes.49 This effect was explored within the Framingham OA Cohort Study.50 In the 556 participants who had complete assessments, a significant threefold to fourfold increase in risk of progression of radiographically determined OA was seen in the middle and low tertiles of vitamin D intake and serum concentration.48 Also, the Study of Osteoporotic Fractures Research Group showed that high serum concentrations of vitamin D protected against both incident and progressive hip OA.51 In more recent results from 2 longitudinal cohort studies the Framingham Offspring cohort (715 subjects) and the Boston Osteoarthritis of the Knee Study (277 participants),52 no association was found between baseline 25(OH)D concentration and radiographic deterioration of joint-space narrowing or cartilage loss. Though the evidence is contradictory for OA, the wide prevalence of vitamin D deficiency and the broad range of important roles played by vitamin D suggest that even OA patients should be encouraged to optimise their vitamin D status.53, 54, 55
Herbal medicines
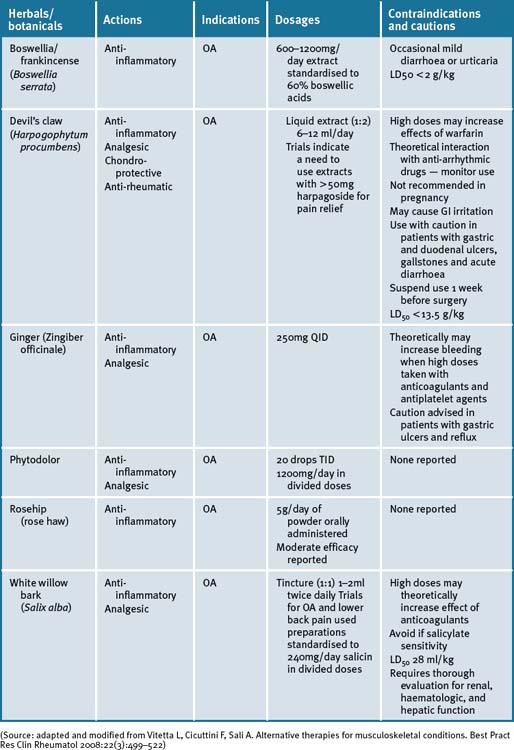
A number of herbal supplements have been investigated for their efficacy in patients with OA (see Table 29.3, at the end of this section).56–60
Green tea extract (Camellia sinensis)
The anti-inflammatory and pharmacological properties of green tea extracts have been attributed to the high content of polyphenols/catechins, of which epigallocatechin-3-gallate (EGCG) predominates.58, 61 The emerging molecular evidence thus far gives strong biological plausibility support to the in vitro observations that catechins extracted from green tea can exhibit both anti-inflammatory and chondro-protective effects and hence may be beneficial for the management of arthritis.61
Cat’s claw (Uncaria tomentosa, Uncaria guianensis)
Extracts of cat’s claw have been shown to possess antioxidant, anti-inflammatory and immuno-modulating properties.57, 58 The most investigated of the active constituents in Uncaria tomentosa extract for immuno-modulating and anti-inflammatory effects are pentacyclic oxindole alkaloids, which are reported to induce an immune regulating factor.58 In a recent review57 the mechanism of cat’s claw action was postulated to be through the inhibition of TNF-alpha. A small study has reported the safe and effective use of cat’s claw in OA of the knee with U guianensis versus placebo.62
Other research groups have documented the safety and pharmacological profile of cat’s claw.57, 63, 64, 65 There is a requisite though for rigorously testing the effectiveness of the recommended doses, which are considered non-toxic, and there are no known contraindications or drug interactions noted at this stage. Until a full pharmacokinetic profile is investigated it would be prudent to avoid its use in women attempting pregnancy, during pregnancy and lactation.57
Devil’s claw (Harpogophytum procumbens)
Harpogophytum procumbens has been shown to be effective for OA in 2 reviews.65, 66 There is little evidence of efficacy for extracts containing less than 30mg/day of the active constituent, harpagoside, and that a correct dose is >50mg/day for OA of the knee and hip. Devil’s claw exhibits cellular signalling modulating activities that down-regulate inflammatory markers.67, 68, 69 A Cochrane review of five randomised clinical trials (RCTs) have reported on the effects of devil’s claw in the treatment of OA.70 Of these, 3 were placebo-controlled and 2 were compared to common pharmaceuticals (diacerhein and phenylbutazone). Three trials demonstrated significant positive results, while 2 studies that employed less than 30mg harpagoside recorded results that were less significant.70 An aqueous extract of devil’s claw (consisting of 60mg harpagoside) was found to be as effective as 12.5mg of rofecoxib for the treatment of acute non-specific lower-back pain in a double-blind pilot RCT.70 Three other trials have also demonstrated efficacy in lower back pain, with 100mg of harpagoside, considered superior, when neurological deficits are present.69, 70 A recent systematic review71 has reported a prevalence of 3% of gastrointestinal complaints in 20 of 28 clinical trials investigated. A few reports of acute toxicity were found (e.g. gastrointestinal complaints, allergies) but there were no reports on chronic toxicity, perhaps due to the low doses utilised in most of the studies.71
Ginger (Zingiber officinale)
The fresh and/or dried roots of Zingiber officinale are reported to possess anti-inflammatory, antiseptic and carminative properties and has been used to treat inflammatory and rheumatic diseases.72 The pungent phenolic constituent of ginger, 6-gingerol, has been shown to inhibit LPS-induced iNOS expression and production of NO in macrophages and to block peroxynitrite-induced oxidation and nitration reactions in vitro.72–74 Cumulative laboratory animal data suggest that 6-gingerol is a potent inhibitor of NO synthesis and effective in inhibiting production of PGE2 and TNF-alpha and COX-2 expression in human synoviocytes by regulating NFκB activation and degradation of its inhibitor IkBa subunit.72
A recent RCT of a combined extract of the herbs Zingiber officinale and Alpinia galanga, comprising 255mg extracted from 2500–4000mg ginger and 500–1500mg galanga rhizome respectively, demonstrated a positive effect on knee OA.75 Clinical trial participants in the ginger/galangal combined extract group experienced a 63% reduction in knee pain on standing versus 50% in the placebo group.75 The highly purified and standardised extract had a statistically significant effect on reducing symptoms of OA of the knee with a high safety profile, and mild GI adverse events in the ginger extract group.75 In a further cross-over RCT, in patients with OA, the ginger extract showed statistically significant efficacy in the first period of treatment before cross-over, however a significant difference was not observed in the study overall.76
Wintergreen (Gaultheria yunnanensis)
Topical natural products that include liniments, balms, creams, gels, oils, lotions, patches, ointments and other products that are applied to the skin are often sought with the intent to provide pain relief for mild arthritis pain that affects only a few joints, as well as to ease sore muscles, back pain and OA.77, 78 No clinical trials are currently available to evaluate these effects. However, in vivo studies have shown that a salicylate fraction isolated from wintergreen has analgesic and anti-inflammatory properties.79 Caution, even with topical products, is required in patients receiving warfarin as adverse interactions and bleeding have been reported to be a risk with its use.80
Phytodolor
Phytodolor is a herbal proprietary product that includes aspen (Populus tremula), goldenrod (Solidago virgaurea) and golden ash (Fraxinus excelsior). Although most of the available literature is German, a recent systematic review of 6 RCTs which included the German studies concluded that Phytodolor reduced the pain associated with rheumatic disorders.80 The dose administered was 30 drops TID for 3 of the trials and 40 drops TID for the remainder, with duration ranging from 2 to 4 weeks.
Boswellia and/or frankincense (Boswellia serrata)
Boswellia serrata (boswellia) is a popular Ayurvedic herb that is purported to exhibit effective analgesic, anti-inflammatory and anti-arthritic activity.81–84 A recent RCT assessed the efficacy, safety and tolerability in 30 patients with OA of the knee over a 16-week period.84 Patients receiving 333mg of boswellia extract containing 40% boswellic acid, 3 times daily, reported a significant decrease in knee pain and swelling, and an associated increase in function and range of movement. Adverse reactions of boswellia therapy were uncommon and included diarrhoea, epigastric pain, and nausea, all of which responded to simple symptomatic management.84 A further RCT compared the same extract with valdecoxib, a non-steroidal anti-inflammatory drug in 66 patients with knee OA over 6 months. This study has a slower onset of action with pain relief persisting for 1 month after ceasing treatment, while valdecoxib acted faster but lasted only during therapy.85
White bark
There is a resurgence of interest in willow bark as a treatment for chronic pain syndromes associated with OA. While white willow (Salix alba) is the willow species most commonly used for medicinal purposes, crack willow (Salix fragilis), purple willow (Salix purpurea), and violet willow (Salix daphnoides) are all salicin-rich species and are available under the label of willow bark. RCTs of short duration have provided evidence of efficacy.86
Historically, Hippocrates was known to prescribe the bark and the leaves of the white willow bark to help relieve pain and fever.86 In 1832, a German chemist produced salicylic acid from salicin, the active ingredient in willow bark. Acetyl salicylic acid was used to produce aspirin and is the more stable form, widely used internationally.86
Rosehip (rose haw)
Recent systematic searches of the literatures,87, 88 have demonstrated that rosehip powder or the seeds of the Rosa canina subspecies had a moderate effect in patients with OA. A study that enrolled 94 patients with OA of the hip or knee in a double-blind placebo-controlled (DBPC) cross-over trial,89 reported that the 47 patients who were given 5g/day of the herbal remedy for a period of 3 months, resulted in a significant reduction in WOMAC pain (P<0.014) as compared to placebo, when testing after 3 weeks of treatment. Furthermore, the clinical data suggested that the herbal remedy not only alleviated symptoms but also reduced the consumption of ‘rescue medication‘ for pain relief.
A recent meta-analysis of the literature identified 3 randomised control trials (RCTs) inclusive of 287 patients over a mean 3-month period and demonstrated rosehip powder significantly reduced OA pain compared with placebo, although long-term studies are required to assess its long-term efficacy and safety.90
Comfrey (Symphytum officinale L)
A recent randomised, double-blind, bi-centre, placebo-controlled clinical trial investigated the effect of a daily application of 6g (3 x 2g) of a commercially available preparation labelled as Kytta-Salbe® f over a 3-week period with patients suffering from painful OA of the knee.91 The results documented suggested that the comfrey root extract ointment was significantly appropriate for the treatment of OA of the knee. Pain was reduced, mobility of the knee improved and quality of life increased.
French maritime pine bark extract
A randomised, double-blind, placebo-controlled trial of 100 patients with mild-moderate knee OA who were treated for 3 months with either 150mg French maritime pine bark extract once daily at meals or by placebo demonstrated reduction of symptoms in the treated group.92 Patients on the herbal extract reported an improvement of WOMAC index, significant alleviation of pain and reduction of analgesia use compared with no effect with placebo. The pine bark extract was well tolerated and postulated to have anti-inflammatory actions.92
Capsaicin (Capsicum frutescens)
A recent systematic review has concluded that topically applied capsaicin has moderate to poor efficacy in the treatment of chronic musculoskeletal or neuropathic pain. Further, that it could be useful as an adjunct or sole therapy for a small number of patients who are unresponsive to, or intolerant of, other treatments.93
Other supplements
Glucosamine
Even though the therapeutic effectiveness of glucosamine treatment on OA has been demonstrated by improved mobility and relief of pain in animal models as well as in RCTs94 its effectiveness as a symptom and disease modifier for OA is still under debate. A recent meta-analysis concluded that the evidence for efficacy for improving symptoms in OA was conflicting and that glucosamine hydrocholoride was not effective.95 A recent study reported that glucosamine sulfate was no better than placebo in reducing symptoms and progression of hip OA.96
A Cochrane review concluded that a specific type of glucosamine sulfate supplement (from Dona, Rotta Pharmaceuticals, Inc) was superior to placebo in the treatment of pain and functional impairment resulting from symptomatic OA.97 Results for the non-Rotta preparation were not statistically significant. The review analysed data from 20 RCTs involving 2570 participants, of which 10 RCTs used the Rotta preparation. A second systematic review reviewed RCTs of at least 1 year’s duration.95 It was reported that glucosamine sulfate may be effective and safe in delaying the progression and improving the symptoms of knee OA. A previous Cochrane review of 16 RCTs had found that glucosamine was effective, however, these included smaller trials with less methodological rigor.98
A concern with most trials of glucosamine sulfate, glucosamine hydrochloride and chondroitin sulfate in the treatment of OA was weak research designs that had been employed.99 The Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) was designed to address these inconsistencies and provide some clarity on the effectiveness of glucosamine (1500mg/day) and chondroitin (1200mg/day) for the treatment of knee pain in OA by employing a rigorous research design.100 The GAIT found that glucosamine and chondroitin sulfate, alone or in combination, did not significantly reduce OA knee pain more than placebo.100 A combination of glucosamine and chondroitin sulfate was found to be effective in a subgroup of patients with moderate-to-severe knee pain (79.2% versus 54.3% for placebo).100 The combined emerging data suggests that glucosamine has a structure-modifying effect. However, debate remains regarding this, largely in relation to methodological issues surrounding outcome measures used in the positive studies.
It has been reported that there is extensive heterogeneity among trials of glucosamine and that this is larger than would be expected by chance. Glucosamine hydrochloride does not appear to be effective.101 Among trials with industry involvement, effect sizes were consistently reported to be higher. The potential explanations may include different glucosamine preparations, inadequate allocation concealment, and industry bias.101
Chondroitin sulfate (CS)
An RCT employing chondroitin sulfate (GS) on 40 patients with tibio-fibular OA of the knee, were allocated to receive 50 intramuscular injections (one injection twice weekly) for 25 weeks. CS had a significantly greater therapeutic effect on all symptoms evaluated.102 No important local or systemic side-effects were noted.102 Favourable effects have been reported in pain reduction and improvement in mobility when CS was given either intra-articularly or orally to elderly patients with joint degeneration.103
A double-blind RCT with 104 patients receiving oral chondroitin-4-sulfate and chondroitin-6-sulfate (CS4 and CS6) at a dose of 800mg/day or placebo for 1 year showed CS4 and CS6 had a beneficial effect, both in terms of clinical manifestations and anatomic progression, in patients with OA of the knee.104 The main efficacy criterion was the Lequesne’s functional index (LFI). Functional impairment was reduced by approximately 50%, with a significant improvement over placebo for all clinical criteria. Tolerance was excellent or good in more than 90% of cases. This study suggests that CS act as structure modulators as illustrated by improvement in the interarticular space visualised on x-rays of patients treated with CS4 and CS6.104
A double-blind RCT of 42 patients with symptomatic OA of the knee examined the effect of 400mg CS twice daily for 1 year.105 After 3 months, joint pain was significantly reduced in the CS group compared to the placebo group. This difference became more pronounced after 12 months. The increase in overall mobility capacity was significantly greater at 6 and 12 months in the CS group than in the placebo group. After 1 year, the mean width of the medial femoro-tibial joint was unchanged from baseline in the CS group, but had decreased significantly in the placebo group. Although no statistical comparison was presented for the change in joint-space width between the 2 groups, the finding suggests the possibility CS treatment may slow the progression of OA.105 The proprietary chondroitin sulfate was studied in a further double-blind RCT of 85 patients with OA of the knee. Participants received Condrosulf® at a dose of 400mg twice daily or placebo for 6 months. Lequesne’s index, spontaneous joint pain, and walking time all decreased progressively in the CS group, with a significant difference in favour of the CS group for each of these parameters.106 In a double-blind RCT parallel group study using either CS 1g/day or placebo on 130 patients for 3 months followed by a 3-month post-treatment period, the CS group experienced greater but non-significant improvement than the placebo group at the treatment endpoint, as measured by the LFI. Improvement became significant in the completer population. In the intent-to-treat population, all variables tended toward greater improvement in the CS than the placebo group. One month after treatment, CS had a significantly higher persistent effect than placebo on the LFI, pain with activity, and other efficacy criteria. Adverse event rates did not differ significantly.107
To assess the clinical efficacy of CS in comparison with the NSAID diclofenac sodium, a multi-centre double-blind RCT, double-dummy study on 146 patients was conducted for 6 months.108 Patients treated with diclofenac showed prompt reduction of clinical symptoms that reappeared, however, after the end of treatment. In the CS group, the therapeutic response appeared later but lasted up to 3 months after the end of treatment. It was concluded that CS had slow but gradually increasing clinical activity in OA, and these benefits lasted a long period after the end of treatment. Shortcomings in these studies were that they involved only a relatively small number of patients and no dose-finding investigations for CS could be found.
A double-blind prospective RCT study of 300 patients given a proprietary registered brand of chondroitin sulfate 800mg/day or placebo for 2 years investigated the structure-modulating properties of CS in gonarthrosis by measuring the modifications in minimum joint space width (JSW), mean thickness, and mean surface of the cartilage in internal femoro-tibial function.109 There was a significant difference, with worsening of the arthritis in the placebo group compared to the CS group. In the group treated with CS, there were no significant variations in any radiological parameters, which remained remarkably stable. The statistical analysis revealed a significant difference in the CS group compared to the placebo group in regard to maintenance of the cartilage analysed, in both the intent-to-treat analysis and also in the per-protocol of analysis subjects who completed the study protocol. It was shown that CS was superior to placebo with regard to stabilisation of minimum JSW of the internal femoro-tibial articular space, the mean thickness, and the surface.109 Hence there is sufficient controlled trial data to support the use of CS in symptomatic OA, having less side-effects than currently used NSAIDs. Chondroitin sulfates appear to have a role in prevention of disease progression. The requisite is that CS be further evaluated in studies of longer treatment duration, with larger numbers of patients, and using well-established measures of function and progression.
A recent meta-analysis reporting on a set of poor to moderate quality, largely heterogeneous (in methodology) trials that made interpretation of the data difficult, concluded that the results were unreliable. Further, the authors concluded that since large-scale, methodologically sound trials indicate that the symptomatic benefit of chondroitin is minimal or nonexistent, that chondroitin cannot be recommended.110
Despite these findings a recent well-performed long-term double-blind study of 622 patients with knee OA, randomised to 800mg of CS or placebo once daily for a 2-year period, demonstrated significant pain relief and radiological improvement by way of demonstrable reduction in minimum JSW loss on X-Rays in the CS group as compared with the placebo group.111
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree