Chapter 2 Organic chemistry
IMPORTANT CONCEPTS IN ORGANIC CHEMISTRY
Essential oil chemistry is a part of organic chemistry, which covers a vast range of compounds. Early ideas suggested that organic compounds were all obtained from either plant or animal sources, i.e. that they were natural products, and arose only through ‘vital forces’ inherent in living cells. This definition is no longer true as a result of modern laboratory synthetic methods. The modern definition of organic chemistry is that it is the chemistry of covalently bonded carbon compounds.
The important points in organic chemistry are outlined below.
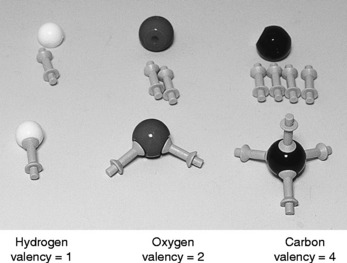
1 Carbon and only very few other elements are involved
The number of organic compounds far exceeds that of inorganic compounds, but very few other elements are involved along with the ubiquitous carbon. Most of them are other nonmetals, most commonly carbon (C) and hydrogen (H), which are always present; oxygen (O), nitrogen (N), sulfur (S) and phosphorus (P), which are commonly found; and chlorine (Cl), bromine (Br), iodine (I) and fluorine (F), which are present in other compounds (see Fig. 2.1).
3 Large molecules are common
Compounds are encountered with molecular formulae such as C20H40, with 20 carbon (C) atoms and 40 hydrogen (H) atoms. Many important biological compounds are what are called macromolecules and are very large. They are polymers made up of many repeating units. For example, cellulose (plant cell wall material) is made up of many linked glucose units and has a molecular weight of 150 000–1 000 000. Insulin (a protein) is made up of 51 amino acid residues and has a molecular weight of about 5700.
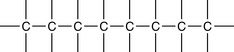
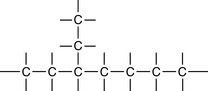
5 Carbon atoms have the ability to bond to other carbon atoms in extended structures
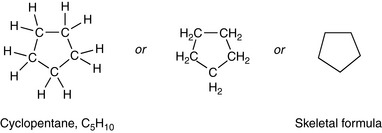
Organic compounds can be found with many forms of ‘carbon skeleton’.
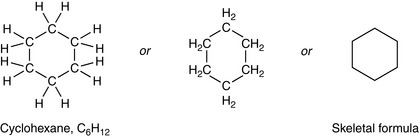
Rings – most commonly with 5 or 6 carbons
A combination of rings and chains
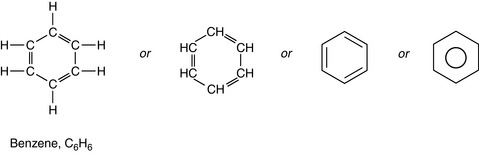
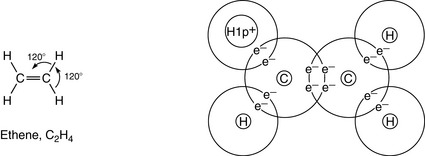
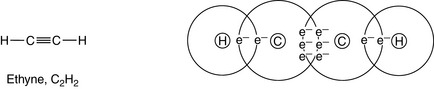
6 Multiple bonding is common
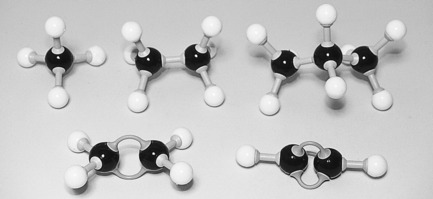
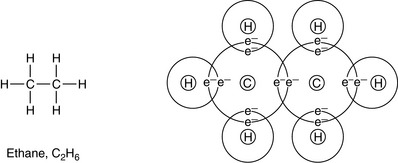
Carbon can form multiple bonds (double or triple bonds; see Ch. 1) between its atoms when forming compounds. This can be shown in some simple hydrocarbons (see Fig. 2.2). In covalent bonds, the sharing of a pair of electrons comprising one electron from each atom involved in the bond is called a single bond and is represented by a single line. The bond is formed by two electrons; for example, in ethane, C2H6:
(In this and the following diagrams, only the outer electrons, taking part in the bonding, are shown; and for simplicity the nucleus of each atom is represented only by the element’s symbol.)