Neuraxial opioids
Treatment drug and doses
Outcomes and conclusion
References
Intrathecal morphine
10–320 μg
Intravenous nalmefene (MOP antagonist)
10–32 μg/kg
Effective
Scratching responses: ↓
Ko and Naughton (2000)
Intrathecal morphine
32 μg
Intramuscular clocinnamox (MOP antagonist)
0.1 mg/kg
Effective
Scratching responses: ↓
Ko et al. (2004)
Intrathecal morphine
32 μg
Subcutaneous butorphanol (mixed KOP/MOP partial agonist)
10–32 μg/kg
Effective
Scratching responses: ↓
Lee et al. (2007)
Intrathecal morphine
10–32 μg
Subcutaneous U-50488H (KOP agonist)
0.1–0.32 mg/kg
Effective
Scratching responses: ↓
Ko et al. (2003b)
Intrathecal morphine
32 μg
Intramuscular nalfurafine (KOP agonist)
0.3–1 μg/kg
Effective
Scratching responses: ↓
Ko and Husbands (2009)
Intrathecal morphine
32 μg
Intramuscular naltrindole (DOP antagonist)
1 mg/kg
Ineffective
No change in scratching responses
Ko et al. (2004)
Intrathecal morphine
50 nmol
Intrathecal N/OFQ (NOP agonist)
10–100 nmol
Ineffective
No change in scratching responses
Ko and Naughton (2009)
Intrathecal morphine
32 μg
Intravenous ondansetron (5HT3 antagonist)
0.1–3.2 mg/kg
Ineffective
No change in scratching responses
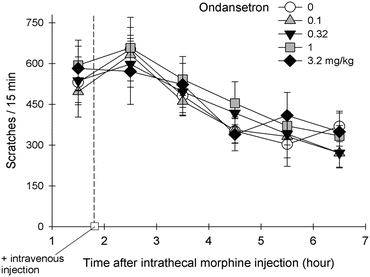
Fig. 1
Intrathecal morphine
32 μg
Intramuscular diphenhydramine (antihistamine)
0.32–3.2 mg/kg
Ineffective
No change in scratching responses
Ko et al. (2004)
Intrathecal morphine
32 μg
Intravenous ketorolac (NSAID)
1–10 mg/kg
Ineffective
No change in scratching responses
Fig. 2
3 Pharmacological Antagonism by Opioid-Related Ligands
3.1 Mu Opioid Receptor Antagonists
As most opioid analgesics used in the clinics are MOP agonists, it is expected that MOP antagonists are effective in treating neuraxial opioid-induced itch in patients (Dominguez and Habib 2013; Ganesh and Maxwell 2007; Kumar and Singh 2013; Waxler et al. 2005). A systematic review of randomized trials involving obstetric patients indicated that intravenous naloxone (0.25–2.4 μg/kg/h) was effective in managing opioid-induced itch (Kjellberg and Tramer 2001). However, MOP antagonists are not widely useful in patients receiving neuraxial opioids for pain relief because MOP antagonists reverse or shorten neuraxial opioid-induced analgesia (Abboud et al. 1990; Cohen et al. 1992; Rawal et al. 1986; Wang et al. 1998).
Antagonist studies in nonhuman primates demonstrate that pretreatment with a single dose of nalmefene (32 μg/kg) was equally potent to block intrathecal morphine-induced itch scratching and antinociception (Ko and Naughton 2000). In this study, the in vivo pK B analysis was used to verify functional receptor populations underlying the actions of intrathecal morphine. The same dose of nalmefene produced approximately tenfold rightward shifts in each subject’s dose–response curves of intrathecal morphine for scratching and antinociception. Accordingly, nalmefene pK B values were similar for both endpoints, indicating that intrathecal morphine-induced scratching and antinociception are mediated by the same MOP population in primates (Ko and Naughton 2000). These findings indicate a narrow window between reversal of itch and analgesia by MOP antagonists and support the clinical findings that MOP antagonists such as naloxone and nalmefene may not be ideal drugs for treating pruritus in obstetric patients. Nevertheless, the MOP antagonist is one of the treatment options for ameliorating cholestatic pruritus, which may be caused by elevated levels of endogenous opioid peptides (Bergasa 2008; Jones and Bergasa 1992).
3.2 Opioid Receptor Partial Agonists
Both nalbuphine and butorphanol are opioid receptor partial agonists that have been used clinically as analgesics with limited abuse liability (Preston and Jasinski 1991). The radioligand binding assay suggests that both drugs have reasonable binding affinity for both MOP and KOP sites in monkey brain membranes, although nalbuphine has a higher selectivity for MOP over KOP (Butelman et al. 1998). In the cell lines expressing MOP or KOP, both drugs displayed low-mid efficacy as measured by the stimulation of [35S]GTPγS binding, i.e., low-mid intrinsic activity (Emmerson et al. 1996; Remmers et al. 1999; Zhu et al. 1997). Interestingly, due to its low efficacy, nalbuphine displays partial MOP agonist actions with its context-dependent agonist/antagonist effects in nonhuman primate behavioral assays (Gerak et al. 1994; Gerak and France 1996). By contrast, butorphanol is characterized as a partial agonist acting at both KOP and MOP sites by diverse in vivo assays in nonhuman primates (Butelman et al. 1995; Lee et al. 2007; Vivian et al. 1999).
Both nalbuphine and butorphanol are effective in alleviating neuraxial opioid-induced itch (Table 2). In particular, systemic nalbuphine between 3 and 10 mg seems effective in decreasing the incidence of pruritus in most of the clinical studies. However, with a high dose of nalbuphine (20 mg), Morgan et al. (1991) did not find pruritus relief by nalbuphine. Butorphanol seems less popular than nalbuphine for treating opioid-induced itch probably due to potential drowsiness following systemic administration. Nevertheless, several studies have shown a decreased incidence of pruritus without other side effects when butorphanol was administered with morphine epidurally in pediatric patients (Bailey et al. 1994; Gunter et al. 2000; Lawhorn et al. 1995; Lawhorn and Brown 1994). A recent systematic review also indicates the potential benefits of using butorphanol to prevent neuraxial morphine-induced itch and decrease pain intensity and postoperative nausea and vomiting without increasing other side effects (Du et al. 2013). Importantly, a pharmacological study demonstrates that butorphanol’s partial agonist actions at both MOP and KOP sites contribute to its antipruritic actions, i.e., low-efficacy ligands antagonize high-efficacy ligand’s action in producing itch sensation (Lee et al. 2007). Compared with MOP antagonists, opioid receptor partial agonists seem to have an advantage for ameliorating itch while retaining analgesia (Dominguez and Habib 2013; Ganesh and Maxwell 2007; Kumar and Singh 2013; Waxler et al. 2005). These observations are in line with preclinical studies demonstrating that butorphanol is effective in alleviating MOP agonist-induced itch without reversing analgesia in nonhuman primates (Lee et al. 2007). Due to butorphanol’s unique pharmacological profile, i.e., partial agonist actions at both MOP and KOP sites, dermatologists are very interested in developing a transdermal formulation of butorphanol for the treatment of chronic itch (Dawn and Yosipovitch 2006; Lim et al. 2008).
Table 2
Summary of clinical studies evaluating the effectiveness of opioid receptor partial agonists in managing neuraxial opioid-induced pruritus in adult patients
Neuraxial opioids | Treatment drugs and doses | Outcomes and conclusion | References |
|---|---|---|---|
Epidural morphine 0.1 mg/kg | Intravenous nalbuphine 0.1 mg/kg | Effective Pruritus score: ↓ | Penning et al. (1988) |
Epidural morphine 5 mg | Intravenous nalbuphine 20 mg | Ineffective No change in the degree of pruritus | Morgan et al. (1991) |
Epidural morphine 5 mg | Intravenous nalbuphine 5 mg | Effective Severity of pruritus: ↓ | Cohen et al. (1992) |
Epidural morphine 5 mg | Intravenous nalbuphine 2.5 mg/h | Effective Pruritus score: ↓ | Kendrick et al. (1996) |
Epidural morphine 3 mg/12 h | Intravenous nalbuphine 60 μg/kg/h | Effective Incidence of pruritus (13 %): ↓ | Wang et al. (1998) |
Epidural morphine 1.5 mg/12 h | Intramuscular nalbuphine 10 mg | Effective Incidence of pruritus (44 %): ↓ Severity of pruritus: ↓ | Liao et al. (2011) |
Intrathecal morphine 200 μg | Nalbuphine (no specified delivery route) 5–10–10 mg, stepwise | Effective VAS score of zero (83 %) | Alhashemi et al. (1997) |
Intrathecal morphine 200 μg | Intravenous nalbuphine 3 mg | Effective Treatment success rate (83 %): ↑ | Charuluxananan et al. (2001) |
Intrathecal morphine 200 μg | Intravenous nalbuphine 4 mg | Effective Pruritus score: ↓ Request for pruritus treatment: ↓ | Charuluxananan et al. (2003) |
Intrathecal morphine 150 μg | Intravenous nalbuphine 2–3 mg | Effective % of successful treatment (87–97 %): ↑ | Somrat et al. (1999) |
Intrathecal fentanyl 50 μg | Intravenous nalbuphine 4 mg | Partially effective Incidence of pruritus (61 %) | Ben-David et al. (2002) |
Epidural morphine 4 mg | Epidural butorphanol 3 mg | Effective % patients treated for pruritus (0 %): ↓ | Lawhorn et al. (1991) |
Epidural morphine 4 mg | Epidural butorphanol 3 mg | Effective Incidence of pruritus (20 %): ↓ | Wittels et al. (1993) |
Epidural morphine 3 mg | Epidural butorphanol 3 mg | Ineffective No change in VAS for pruritus | Gambling et al. (1994) |
Epidural morphine 60 μg/kg | Epidural butorphanol 30 μg/kg | Effective Severity of pruritus: ↓ | Bailey et al. (1994) |
Intrathecal morphine 150 μg | Intravenous butorphanol 2 mg | Ineffective No change in the intensity of pruritus | Sakai et al. (2001) |
Intrathecal morphine 100 μg | Intravenous butorphanol Bolus 1 mg with 0.2 mg/h | Effective Incidence of pruritus (13 %): ↓ | Wu et al. (2012) |
3.3 Kappa Opioid Receptor Agonists
Numerous preclinical and clinical studies have indicated that KOP is a viable therapeutic target for potential antipruritics (Cowan and Gmerek 1986; Ko et al. 2003b; Kumagai et al. 2010, 2012). Original studies in rodents showed that systemic administration of KOP agonists inhibited scratching activity evoked by pruritogens such as bombesin-related peptides (Gmerek and Cowan 1983, 1984). In particular, KOP agonists inhibited scratching behavior without interfering with locomotor activity in rodents (Inan et al. 2009; Togashi et al. 2002; Wang et al. 2005). Recent studies have identified a subset of inhibitory interneurons regulating itch in the dorsal horn of mouse spinal cord (Ross et al. 2010). It will be important to investigate the role of KOP modulating these inhibitory interneurons. Furthermore, pharmacological studies in nonhuman primates have demonstrated that KOP agonists, at nonsedating doses, can attenuate intrathecal morphine-induced scratching without affecting antinociception (Ko and Husbands 2009; Ko et al. 2003b). These findings facilitated the development of a KOP agonist, nalfurafine, as an antipruritic. To date, two clinical trials have reported that nalfurafine is a safe and effective antipruritic in hemodialysis patients suffering from uremic pruritus (Kumagai et al. 2010, 2012).
KOP agonists produce several effects opposite to those of MOP agonists in primates. For example, MOP agonists produce euphoria, whereas KOP agonists produce dysphoria (Kumor et al. 1986; Walsh et al. 2001); MOP agonists produce antidiuretic effects, while KOP agonists produce diuresis (Peters et al. 1987; Weiskopf et al. 1987). Although there is no selective KOP agonist approved for treating neuraxial opioid-induced itch, it seems promising to develop KOP-related ligands, especially mixed KOP/MOP agonists for this purpose or as spinal analgesics. Clinically used mixed KOP/MOP agonists such as butorphanol and pentazocine have a low incidence of pruritus and are effective in treating spinal morphine-induced itch (Abboud et al. 1989; Ackerman et al. 1989; Lawhorn et al. 1991; Tamdee et al. 2009). In addition, butorphanol produces neither euphoria nor dysphoria in humans and it does not cause diuresis (Butelman et al. 1995; Dershwitz et al. 1991). These findings strengthen the notion that mixed KOP/MOP agonists may have a therapeutic advantage over selective MOP agonists. It will be important to further develop novel opioid agonists with dual actions at both KOP and MOP sites with different degrees of intrinsic efficacy and advance the medicine of neuraxial opioids.
4 Pharmacological Antagonism by Non-Opioid Ligands
4.1 Serotonin 5-HT3 Receptor Antagonists
The effectiveness of a 5-HT3 receptor antagonist, ondansetron, in treating neuraxial opioid-induced itch varies across different clinical studies (Table 3). Several studies showed that intravenous ondansetron (4–8 mg) was effective in decreasing the incidence of pruritus in patients receiving either epidural or intrathecal morphine, fentanyl, or combination of MOP agonists. However, several other studies concluded that ondansetron was ineffective in treating itch in most of the patients receiving intrathecal fentanyl or combination of MOP agonists (Bonnet et al. 2008). It will be important to investigate whether fentanyl, sufentanil, or a combination of MOP agonists elicits a higher intensity of itch as both fentanyl and sufentanil have been characterized in the agonist stimulation of [35S]GTPγS binding as full MOP agonists with higher intrinsic activity as compared to morphine (Emmerson et al. 1996).
Table 3
Summary of clinical studies evaluating the effectiveness of a 5-HT3 receptor antagonist, ondansetron, in managing neuraxial opioid-induced pruritus in adult patients
Neuraxial opioids | Treatment drug and doses | Outcomes and conclusion | References |
|---|---|---|---|
Epidural morphine, 2 mg Intrathecal morphine, 0.2 mg | Intravenous ondansetron 8 mg | Effective Success rate (70 %): ↑ | Borgeat and Stirnemann (1999) |
Epidural morphine 3 mg | Intravenous ondansetron 4 mg | Effective Incidence of pruritus (28 %): ↓ | Tzeng et al. (2003) |
Intrathecal sufentanil 2.5 μg and morphine 100 μg | Intravenous ondansetron 8 mg | Ineffective No change in the frequency and severity of pruritus | Yazigi et al. (2002) |
Intrathecal morphine 160 μg and fentanyl 15 μg | Intravenous ondansetron 8 mg | Ineffective No change in the incidence of pruritus | Sarvela et al. (2006) |
Intrathecal morphine 250 μg | Intravenous ondansetron 4 mg | Effective Incidence of pruritus (34 %): ↓ | Iatrou et al. (2005) |
Intrathecal morphine 200 μg | Intravenous ondansetron 4–8 mg | Effective Request for pruritus treatment: ↓ | Charuluxananan et al. (2003) |
Intrathecal morphine 200 μg | Intravenous ondansetron 4 mg | Effective Treatment success rate (80 %): ↑ | Charuluxananan et al. (2000) |
Intrathecal morphine 200 μg | Intravenous ondansetron 4 mg Orally disintegrating tablets 8 mg | Effective Incidence of pruritus (56–66 %): ↓ | Pirat et al. (2005) |
Intrathecal morphine 150 μg | Intravenous ondansetron 0.1 mg/kg | Effective Incidence of pruritus (25 %): ↓ | Yeh et al. (2000) |
Intrathecal fentanyl 25 μg | Intravenous ondansetron 4–8 mg | Ineffective No change in the incidence and severity of pruritus | Wells et al. (2004) |
Intrathecal fentanyl 25 μg | Intravenous ondansetron 8 mg | Effective Incidence of pruritus (39 %): ↓ | Gurkan and Toker (2002) |
Intrathecal fentanyl 25 μg | Intravenous ondansetron 8 mg | Effective Incidence of pruritus (6 %): ↓ | Gulhas et al. (2007) |
Intrathecal fentanyl 15 μg | Intravenous ondansetron 8 mg | Ineffective No change in the incidence of pruritus | Browning et al. (2013) |
Intrathecal fentanyl 10 μg | Intravenous ondansetron 4–8 mg | Ineffective No change in the incidence and severity of pruritus | Korhonen et al. (2003) |
Intrathecal sufentanil 10 μg | Intravenous ondansetron 8 mg | Ineffective No change in the incidence and severity of pruritus | Waxler et al. (2004) |
The exact mechanism for ondansetron to alleviate itch is unknown. Although the 5-HT3 receptors can be identified in the spinal cord of rodents and primates (Laporte et al. 1996; Waeber et al. 1988), there is no anatomical evidence for the co-localization of the 5-HT3 receptor with MOP in the spinal cord or functional evidence for the interaction between the 5-HT3 receptor and MOP in any animal models. Since patients with cholestatic pruritus have elevated levels of endogenous opioids, there were several randomized controlled trials exploring the effects of ondansetron (Jones et al. 2007). It was concluded that ondansetron has negligible effect on cholestatic or uremic pruritus based on a recent systematic review (To et al. 2012). Figure 1 illustrates the effects of ondansetron on intrathecal morphine-induced scratching in monkeys. Intrathecal administration of morphine (32 μg) elicited profound scratching responses (i.e., ~600 scratches within a 15-min bin/time sampling) in rhesus monkeys (n = 8) (unpublished data from the Ko lab). Intravenous ondansetron (0.1–3.2 mg/kg) was given approximately 2 h after subjects received intrathecal morphine. Within these doses tested herein, ondansetron was ineffective in attenuating intrathecal morphine-induced scratching. A higher dose of ondansetron (10 mg/kg) caused extrapyramidal reactions in monkeys (i.e., involuntary head jerking, both legs were rigid and were in extensor spasm) which led to the termination of experiments.