Fig. 1
Structure of natriuretic peptides. A comparison of the primary structure (table) of mouse, human, and rat natriuretic polypeptides reveals that Nppa displays greater sequence conservation than Nppb. Despite these differences, both Nppa and Nppb activate the same receptor; Npr1. Amino acids are color coded to reflect their characteristics. The structure of the natriuretic peptides is schematized (to the right) to highlight the core conserved ring that forms the key structural element of these transmitters. Only amino acid residues that have high sequence conservation between species and between Nppa and Nppb are colored. The cysteine residues and their critical disulfide bond are marked in yellow. The diagram also illustrates that the N-terminal and C-terminal loops exhibit low sequence identity and are not thought to be important determinants in receptor binding
Table 1
Table of the major itch neurotransmitters and their receptors
Receptor | Gene name | Agonist | Antagonist | Transducer |
|---|---|---|---|---|
Natriuretic peptide receptor A | Npr1 | Nppa (Atlas et al. 1985) Nppb (Tse et al. 2001) | Anantin (Wyss et al. 1991) | Guanylyl cyclase (Garbers and Lowe 1994) |
Gastrin-releasing peptide receptor | Bb2 | GRP (Jensen et al. 2008) | BIM 189 (Coy et al. 1990) | Gq/G11 family (Hellmich et al. 1997) |
Somatostatin receptor 2 | Sstr2 | Sst (Kardon et al. 2014) Octreotide (Raynor et al. 1993) | CYN-154806 (Bass et al. 1996) | Gi/Go family (Hou et al. 1994) |
Kappa-opioid receptor | Opkr1 | Dynorphin (Kardon et al. 2014) Nalfurafine, U-50,488 (Morgan and Christie 2011) | Nor BNI, 5′-GNTI (Morgan and Christie 2011) | Gi/Go family (Dhawan et al. 1996) |
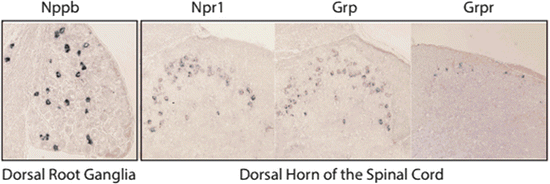
Fig. 2
Expression pattern of itch-specific neurotransmitters and their receptors. In situ hybridization shows the neurons which express the transmitters Nppb and Grp and their respective receptors, Npr1 and Grpr. Nppb is expressed in about 6 % of DRG neurons that have small diameters. The receptor for Nppb, Npr1, is expressed in dorsal horn neurons. Likewise, Grp and Grpr are also expressed in the superficial dorsal lamina of the spinal cord
2 Excitatory Neurotransmission in the Spinal Cord
The dorsal horn of the spinal cord is well known to not only act as the conduit of pruritic, painful, and thermal input but is also known to be a site where somatosensory afferent information is integrated. An indication of the complexity of the circuits in the spinal cord is provided by the demonstration of a large number of transmitters that are present in this tissue (Su et al. 2014). Despite this apparent overwhelming complexity, significant headway has been made in unraveling the transmitters and cellular mechanisms involved in itch transmission.
The neurons which express the primary itch transmitter receptor, Npr1, are thought to be the first element in the itch neural circuit in the spinal cord (Mishra and Hoon 2013). It was noticed that ablation of these neurons (using Nppb-saporin neurotoxin) did not eliminate Grp-induced itch. This result is consistent with the transmitter Grp being downstream of Nppb. Furthermore, in mice lacking Nppb, intrathecal administration of Grp elicited normal itch responses. What is more, antagonist blockade of Grpr or elimination of Grpr neurons inhibited equally Grp- and Nppb-elicited itch behavior, concordant with a linear circuit for itch in the dorsal horn of the spinal cord (Fig. 3). By assaying the expression of Grp, it was revealed that Grp is present at high levels in interneurons in the dorsal horn of the spinal cord (Fig. 2) (Fleming et al. 2012; Li et al. 2006; Mishra and Hoon 2013; Wang et al. 2013; Xu et al. 2013). To better define the neurons that express Grp, mice in which Npr1-cells are ablated were examined to see if the removal of these neurons leads to a concomitant reduction of Grp-expressing cells. As expected for the co-expression of Grp and Npr1, there was a dramatic reduction of Grp-expressing spinal cord neurons after Nppb-saporin treatment. Double-labeling experiments further confirmed that Grp and Npr1 are co-expressed (Mishra and Hoon 2013). The Grp/Npr1 class of neurons in addition to being stimulated by Nppb is likely activated by glutamate (Koga et al. 2011). Therefore, there is strong evidence that Grp is downstream of Nppb and that Npr1-spinal cord interneurons are activated by the presynaptic release of Nppb and glutamate from DRG afferents (Fig. 2). Nevertheless, the importance of Grp in the itch pathway is undisputed; ablation of Grpr neurons, elimination of the Grpr receptor itself, or administration of Grpr antagonist causes the selective loss of itch responses (Sun and Chen 2007; Sun et al. 2009). A recent pair of papers confirms the crucial roles of Grpr- and Grp-expressing interneurons in itch (Wang et al. 2013; Xu et al. 2013). The conditional deletion of the testicular orphan nuclear receptor 4, TR4, results in the loss of several types of dorsal horn neurons and also causes a dramatic reduction in responsiveness to itch-inducing agents. TR4 mutant mice have normal numbers of primary sensory neurons and projection neurons and only show deficits in the number of excitatory dorsal horn neurons. The neurons lost in TR4 conditional knockouts include those expressing Grp and Grpr. Using a different genetic strategy, another study examined the consequence of the loss of the T-cell leukemia homeobox 3 gene, Tlx3, in dorsal horn neurons (Xu et al. 2013). Tlx3 conditional knockout mice lack a subset of spinal cord excitatory neurons in laminae I and II, including those which express Grp and Grpr. These mutant mice, like TR4 knockout animals, have lost responses to itch-inducing agents. However, as both these mutant mice have reduced responses to other somatosensory stimuli, assessment of the specificity of Grp and Grpr neurons and where they fit in itch-circuit models cannot be directly deduced from these studies.
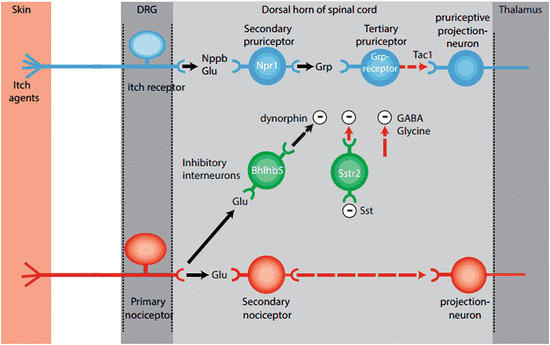

Fig. 3
Schematic of the itch circuit. Schematic representation of the distribution and function of itch-selective transmitters and their neuronal targets illustrates the simplicity of the itch circuit. Excitatory pathway (blue-colored cells): (1) glutamatergic and Nppb excitatory input from pruritic neurons activates Npr1-expressing spinal cord neurons. (2) Npr-1-expressing neurons release Grp which in turn activate tertiary pruriceptive neurons that express the Grpr receptor. (3) The transmitter used by Grpr-expressing neurons has not yet been identified; however, substance P (Tac1) is believed to play a role in the activation of the last step in the spinal cord itch circuit and the stimulation of spinothalamic projection interneurons. Inhibitory pathway (green-colored neurons): (1) glutamatergic afferents of nociceptors (red-colored neurons) stimulate Bhlhb5-inhibitory neurons. (2) Bhlhb5-expressing neurons release dynorphin (Pdyn) which activates Kappa-opioid receptors in postsynaptic membranes of unknown pruritic pathway neurons. (3) Somatostatin (Sst) from dorsal horn or DRG neurons activates and in turn inhibits neurons which express the Sstr2 receptor. Since Sstr2 leads to reduced cell activity, Sst-mediated activation of these neurons causes disinhibition and therefore potentiates itch behavior. The inhibitory neurotransmitters GABA and glycine are also involved in reducing itch responses. The segments of the pathway that are unknown or controversial are marked in red
Another excitatory neuropeptide that may be used by the spinal cord itch pathway is substance P (Stander et al. 2010; Trivedi and Bergasa 2010). Although the gene that encodes substance P, Tac1, is not necessary for itch responses, several studies which used substance P antagonists reported that it can attenuate pruritic behavior (Akiyama et al. 2014; Cuellar et al. 2003). In addition, the ablation of substance P receptor-expressing (NK-1) dorsal horn neurons with substance P-saporin also greatly reduced responses elicited by pruritic compounds (Carstens et al. 2010). Lastly, spinothalamic projection neurons which convey itch signals from the spinal cord to the brain are immunopositive for the substance P receptor, NK-1 (Todd et al. 2000). The ablation of NK-1-expressing neurons causes a deficit in responses to painful stimuli as well as itch, which together with electrophysiological results has been interpreted to show convergence of sensory information in spinal cord projection neurons (Davidson et al. 2009, 2012; Mantyh et al. 1997).
3 Inhibitory Transmission in the Spinal Cord
Thermal, painful, and mechanical stimuli can inhibit itch (Bromm et al. 1995; Yosipovitch et al. 2007). An important clue to the mechanism for the inhibition of itch by counter-stimuli was obtained by examining the effect of silencing glutamate transmission from nociceptive neurons (Lagerström et al. 2010; Liu et al. 2010). By selectively abolishing glutamate neurotransmission (by eliminating VGlut2) in various classes of nociceptors, including those which express the voltage-gated sodium channel Nav1.8, pruritogen-induced itch was markedly increased, while nociceptive responses were greatly reduced. As a note of caution, a comparable but not identical study in which other somatosensory-specific conditional VGlut2 mice were examined showed no change in itch behavior in mutant animals (Scherrer et al. 2010). At present it is not possible to reconcile these differing results. Nevertheless, an explanation for the novel itch behavior described for Nav1.8 conditional VGlut2 mice was proposed in which these animals lose inhibition of pruritic input (Lagerström et al. 2010; Liu et al. 2010). It was suggested that normally tonic pruritic input is silenced by paired inhibitory nociceptive input. A model to account for this explanation posits that a class of inhibitory neurons is activated by nociceptive input; these cells in turn normally inhibit itch signals (Fig. 3). Indeed, this model is supported by mutant mice that lack a specific class of inhibitory neurons (Bhlhb5 cells) and display a similar spontaneous itch phenotype to that of conditional VGlut2 mutants (Ross et al. 2010). A search for the mechanism by which Bhlhb5 neurons inhibit itch revealed that they likely use the inhibitory transmitter, dynorphin, a kappa-opioid receptor agonist (Table 1). Initially, it was shown that the kappa-opioid receptor agonist nalfurafine inhibits scratching (Inan and Cowan 2006; Kardon et al. 2014), suggesting that kappa-opioid receptors are part of the inhibitory pathways for itch. Recently, it was shown that the endogenous kappa-opioid receptor agonist, dynorphin, is expressed in Bhlhb5 neurons (Kardon et al. 2014). Grp-induced itch is inhibited by nalfurafine suggesting that dynorphin probably inhibits either Grpr neurons or cells further downstream in the itch pathway (Inan et al. 2011; Kardon et al. 2014). These results establish that dynorphin is an important negative modulator of itch signals. However, it must be noted that dynorphin knockout mice exhibit no itch phenotype, suggesting that there may be functional redundancy in the transmitters for inhibition or there is development plasticity which makes the role of dynorphin dispensable (Kardon et al. 2014). In addition to dynorphin, the neuropeptide somatostatin (Sst) has been reported to induce itch-scratching responses (Kardon et al. 2014). A receptor for Sst, Sstr2 (Table 1), is found in many inhibitory neurons in the dorsal horn of the spinal cord (Polgar et al. 2013). Bhlhb5 neurons co-express the Sstr2 receptor and can be hyperpolarized by application of Sst, suggesting that Sst may induce scratching by disinhibiting Bhlhb5 neurons. However, Sstr2 receptors are expressed in other classes of spinal cord interneurons, as well as in DRG cells, and an alternate explanation of the inhibitory effects of Sst is that it triggers responses in these other classes of cells (Polgar et al. 2013; Shi et al. 2014; Takeda et al. 2007; Yasaka et al. 2010). In addition, the effects of Sst on nociception are well characterized, which, given the interplay between itch and nociception input, could account for the induction of itch by Sst. Lastly, other inhibitors of itch are the neurotransmitters GABA and glycine (Akiyama et al. 2011). At present the exact source and cellular substrates for these transmitters are unknown. Cumulatively, we are beginning to see the outline of the inhibitory transmitters and interneurons that are responsible for the cross talk between different sensory modalities which is crucial for the sentient perception of our environment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


