In addition to fetal chromosome and genome analysis, the concentration of alpha-fetoprotein (AFP) can be assayed in amniotic fluid to detect open neural tube defects (NTDs) (see Chapters 8 and 14). AFP is a fetal glycoprotein produced mainly in the liver, secreted into the fetal circulation, and excreted through the kidneys into the amniotic fluid. AFP enters the maternal bloodstream through the placenta, amniotic membranes, and maternal-fetal circulation. It can therefore be assayed either in amniotic fluid (amniotic fluid AFP [AFAFP]) or in maternal serum (maternal serum AFP [MSAFP]). Both assays are extremely useful for assessing the risk for an open NTD but also for other reasons (see later discussion).
AFP concentration is measured by an immunoassay, a relatively simple and inexpensive method that can be applied to all amniotic fluid samples, regardless of the specific indication for the amniocentesis. To interpret an AFAFP, one compares the level to the normal range for each gestational age. If the AFAFP level is elevated (relative to the normal range for that particular gestational age), one must look for an open NTD as well as for causes other than an open NTD. Factors potentially leading to abnormally high concentrations of AFP in amniotic fluid are shown in Table 17-1. When the AFAFP assay is used in conjunction with ultrasonographic scanning at 18 to 19 weeks’ gestation, approximately 99% of fetuses with open spina bifida and virtually all fetuses with anencephaly can be identified.
If amniocentesis is performed for any reason, both the concentration of AFP in the amniotic fluid and a chromosome analysis of amniotic fluid cells are determined to screen for open NTDs and chromosomal and other genomic abnormalities, respectively. Other tests are performed only for specific indications.
Complications.
The major complication associated with midtrimester amniocentesis at 16 to 20 weeks of gestation is a 1 in 300 to 1 in 500 risk for inducing miscarriage over the baseline risk of pregnancy loss of approximately 1% to 2% for any pregnancy at this stage of gestation. Other complications are rare, including leakage of amniotic fluid, infection, and injury to the fetus by needle puncture. Early amniocentesis performed between 10 and 14 weeks is no longer recommended because of an increased risk for amniotic fluid leakage, a threefold increased risk for spontaneous abortion, and an approximately sixfold to sevenfold increased risk for talipes equinovarus (clubfeet), over the 0.1% to 0.3% population risk. Early amniocentesis has now been replaced by chorionic villus sampling (see next section).
Chorionic Villus Sampling
CVS involves the biopsy of tissue from the villi of the chorion transcervically or transabdominally, generally between the 10th and 13th weeks of pregnancy (see Fig. 17-1B). Chorionic villi are derived from the trophoblast, the extraembryonic part of the blastocyst (Fig. 17-2), and are a ready source of fetal tissue for biopsy. As with amniocentesis, ultrasonographic scanning is used before CVS to determine the best approach for sampling.
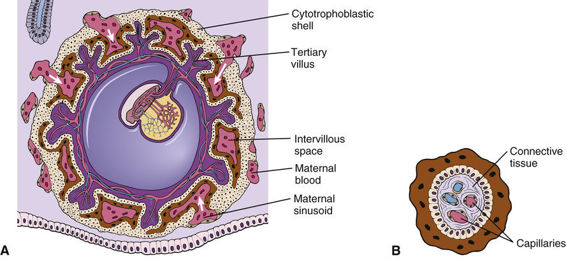
The major advantage of CVS compared with midtrimester amniocentesis is that CVS allows the results to be available at an early stage of pregnancy, thus reducing the period of uncertainty and allowing termination, if it is elected, to be performed in the first trimester. However, unlike after amniocentesis, AFAFP cannot be assayed at this stage. Evaluation for a possible open NTD thus must be done by other methods, including MSAFP screening, amniocentesis for AFAFP, and ultrasonography.
The success of chromosome analysis by karyotype or CMA is the same as with amniocentesis (i.e., more than 99%). However, approximately 1% of CVS samplings yield ambiguous results because of chromosomal mosaicism (including true mosaicism and pseudomosaicism; see later); in these situations, follow-up with amniocentesis is recommended to establish whether the fetus has a chromosomal abnormality.
Complications.
In prenatal diagnostic centers experienced in performing CVS, the rate of fetal loss is only slightly increased over the baseline risk of 2% to 5% in any pregnancy of 7 to 12 weeks of gestation and approximates the 1 in 300 to 1 in 500 risk seen with amniocentesis. Although there were initial reports of an increase in the frequency of birth defects, particularly limb reduction defects, after CVS, this increase has not been confirmed in large series of CVS procedures performed after 10 weeks of gestation by experienced physicians.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



