Fig. 1
The overlap of brain activations induced by histamine itch (in green) and by cowhage itch (in blue) illustrates the regions coactivated (in red) and distinct areas activated separately by the two itch pathways. Standard Talairach space coordinates. The color tones displayed correspond to Z score values as shown in the color bar. ACC anterior cingulate cortex, PCC posterior cingulate cortex, SPL superior parietal lobule, M1 primary motor cortex, S1 primary somatosensory area, SMG supramarginal gyrus, MTG middle temporal gyrus, IPL inferior parietal lobule, S2 secondary somatosensory area, VPL ventral posterior lateral nucleus (of thalamus) (can be appended separately; from NeuroImage 59(4) 2012)
7 Brain Processing of Chronic Itch
A few neuroimaging studies have been performed in patients with chronic itch. These studies have revealed altered function (Schneider et al. 2008; Ishiuji et al. 2009; Papoiu et al. 2014); one study also demonstrated altered structure in the frontolimbic regions compared to healthy controls.
In atopic dermatitis, which is the most common skin disease causing chronic itch, activation of the ACC and DLPFC is directly correlated with disease severity (as measured by the Eczema Area and Severity Index (EASI) score, a standardized validated clinical tool). Intensity of histamine-induced itch correlates with activations in the ACC and insula. Overall, the pattern of association between activation and perceived itch intensity is different in healthy volunteers (Schneider et al. 2008; Ishiuji et al. 2009; Yosipovitch group, unpublished). The distinction between the patterns evoked by histamine and cowhage itches, clearly identified in healthy individuals, appears to be blurred in chronic itch diseases (ESRD and AD). Recently, an investigation of structural and functional perfusion differences between ESRD patients with chronic pruritus and healthy individuals found a significant thinning of the gray matter in the thalamus, insula, ACC, precuneus, and caudate body (areas involved in itch processing) in the ESRD patients. Assessment was by voxel-based morphometry (VBM), a technique that quantitates gray matter densities. These MR-based changes were not demonstrated in two chronic itch states: atopic eczema and psoriasis (unpublished data). In pruritic patients with ESRD, persistent perfusion increases at baseline in the insula, ACC, claustrum, amygdala, hippocampus, and NAc—areas that are known to be highly activated by itch (Fig. 2). Interestingly, baseline brain perfusion in chronic itch of atopics and psoriatics does not differ from that in healthy subjects. These results further suggest that chronic itch states differ in brain imaging patterns. Moreover, the processing of cowhage itch appeared altered in ESRD, while no significant differences could be demonstrated in the processing of histamine-induced itch. In ESRD pruritus, multiple brain activations appear to work either directly or are inversely correlated with perceived itch intensity, suggesting a dual modulation of itch perception. These unique features can be facilitated by the reduced gray matter thickness in ESRD affecting critical areas involved in itch processing, thus revealing a form of neocortical plasticity. As with chronic pain, central neuronal remodeling occurs (Parise et al. 2014). In ESRD, it appears that the PAR2-mediated itch pathway is already overstimulated, in association with an overexpression of PAR2 in the skin. This may lead to a tonic inhibition of cortical processing of acute cowhage itch when induced in the preexistent context of ESRD.
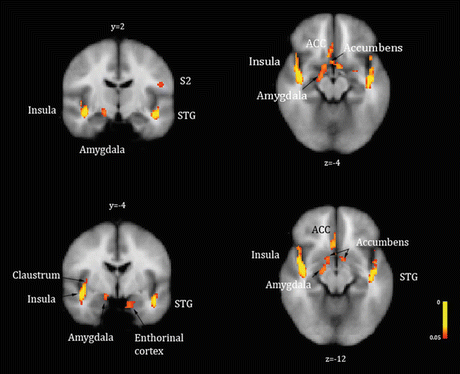

Fig. 2
Baseline state (resting) perfusion increases or brain activations identified in ESRD patients with chronic pruritus in significant contrast with healthy volunteers. Brain perfusion was higher at baseline in ESRD patients with chronic pruritus compared to healthy individuals in the insula, claustrum, ACC, amygdala, entorhinal cortex, and subcallosal gray matter—nucleus accumbens. Arterial spin labeling fMRI; p < 0.05. STG superior temporal gyrus, S2 secondary somatosensory area, ACC anterior cingulate cortex
A central “top-to-bottom” inhibition of itch is drawn from the parallel descending pathway for suppression of pain and proposes that the PAG modulates the activity of spinal interneurons. According to this model, descending inputs directed toward itch receptive neurons in the dorsal horn exert an inhibitory action, effectively silencing them (Carstens 1997; Davidson and Giesler 2010). Another possibility is that the cortical projection of itch information into S1/S2 may be inhibited via cortico-cortical inhibitory loops, in a similar fashion to mechanisms that have been known to operate in chronic pain (Henry et al. 2011). Our recent findings in ESRD patients possibly suggest that a tonic inhibition may be exerted at the neocortical level to selectively limit the receptive fields for PAR2-mediated itch processing in S1, precuneus, and insula. These findings are of significant interest because they offer insight into mechanisms the brain may employ to process and modulate itch sensation. Contrary to a widely accepted paradigm in the neuroimaging literature, it is thus possible that a higher intensity itch does not necessarily translate into a higher or more extensive activation of the cerebral cortex. Moreover, longitudinal studies from patients with chronic pain, which shares many similarities with chronic itch, suggest that abnormalities in gray matter densities within brain regions such as the ACC and insula can resolve after treatments that reduce the symptom (Rodriguez-Raecke et al. 2009).
8 Craving for Itch Relief and Its Cerebral Mechanisms
The sensation of itch and the immediate craving for itch relief manifested as the urge to scratch are inseparable. Four recent fMRI studies have investigated the cerebral processing of scratching. In three of them, experimenters scratched the subjects’ skins using brushes and copper plates (Yosipovitch et al. 2007, 2008; Vierow et al. 2009; Mochizuki et al. 2014). In the fourth one, visualized active scratching provided a more robust scratch response (Papoiu et al. 2014). The observed brain regions common to these studies were the PFC, ACC, insula, secondary S2, and cerebellum (Fig. 3). The significant activations of the PFC and insula during scratching are interesting since these regions, in particular the PFC, are less sensitive to other tactile stimuli such as a vibrotactile stimulus (Gelnar et al. 1999; Seitz and Roland 1992; Coghill et al. 1994; Golaszewski et al. 2002), Hagen and Pardo 2002; Burton et al. 2004). Several clinical studies on patients with addictive behaviors have also demonstrated the importance of the PFC in motivation and reward. Reward circuits have been linked with the pleasurability of scratching and may play an important role in itch inhibition, as well as in the formation of “vicious” itch–scratch cycles. Recent findings suggest that the reward system of the midbrain, more specifically the ventral tegmentum (VTA), and substantia nigra, as well as the NAc, may play a role in the urge to scratch and the subsequent satisfaction (pleasure) derived from scratching, via connections with the insula, ACC, and striatum (Papoiu et al. 2014; Mochizuki et al. 2013, 2014). The involvement of VTA and NAc underscores the addictive nature of the itch–scratch cycle and also suggests a role for the dopaminergic system in itch relief (Papoiu et al. 2013, 2014).
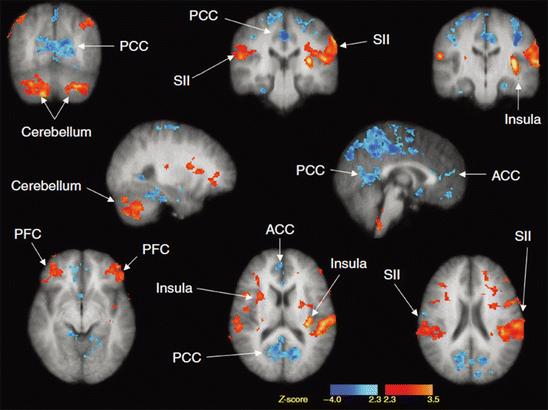

Fig. 3
Brain regions activated by scratching the skin. Red and blue regions were where neural activity was significantly increased and decreased during scratching, respectively. PCC posterior cingulate cortex, SII secondary somatosensory cortex, ACC anterior cingulate cortex, PFC prefrontal cortex
9 Role of the PAG in Itch Inhibition
The PAG has been considered the major descending inhibitory control of pain (Millan 2002). A PET study showed increased PAG activity, while itch sensation was reduced, by applying pain stimuli (Mochizuki et al. 2003). Thus, it is likely that descending inhibitory control has the potential to inhibit itch sensation. However, our recent fMRI studies of scratching did not show activation of the PAG; in fact, deactivation of the PAG was noted with high Z scores. Additionally, there was significant activation of the ventral tegmentum (which is closely related to the PAG) during scratching an itch. Thus, more precise investigations using electrophysiological techniques with animals, and fMRI with higher spatial resolution (e.g., 7 T), will be necessary to clarify whether descending PAG inhibitory control is associated with itch inhibition by scratching.
10 Modulation of Itch Targeting the Brain
Currently, there are no published studies on the effect of antipruritic drugs against itch-related changes in brain wiring. We recently demonstrated that butorphanol, a kappa opioid agonist and mu opioid antagonist, known to exert antipruritic effects in the spinal cord (Dawn and Yosipovitch 2006) completely suppressed the itch induced experimentally with histamine (Papoiu et al. 2015). The functional MRI data showed that, in comparison with the placebo, butorphanol produced a bilateral deactivation of the claustrum, insula, and putamen, areas described to be activated during itch processing. The inhibition of histamine itch by butorphanol was paralleled by well-defined, significant activations which mapped to nucleus accumbens bilaterally and to a subcallosal gray matter area on the midline consistent with the location of septal nuclei. Our results indicate that the antipruritic action of butorphanol is mediated by these two formations, known to express a high density of κ opioid receptors (Peckys and Landwehrmeyer 1999; Peckys and Hurd 2001) on which it is likely the κ opioid agonist, butorphanol, acts. This is the first clear identification of discrete structures within the human brain capable of exerting itch suppressions upon opioid activation.
Another study assessed the effect of acupuncture on itch-evoked activation and demonstrated reduced activation in the insula, putamen, and premotor and prefrontal cortical areas (Napadow et al. 2012). An overactive limbic system (anterior cingulate cortex–amygdala–nucleus accumbens) may reflect a more intense, unbalanced craving for itch relief, accompanied by activations in the insula (as seen in ESRD pruritus at baseline) as well as an increase in acute itch induction in atopic eczema itch and psoriatic itch. This leads to the amplification of compulsive scratching behavior and more distress. Therefore, addressing the emotional and psychological suffering by targeting these areas is a cornerstone for building a successful therapy for pruritus. Brain areas involved in self-awareness (precuneus) and self-perception (insula) are involved, confirming the observation that itch is a very intrusive and disturbing sensation, perturbing the well-being of the person.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


