No. of subjects
37
Male/female
11/26
Age (years; mean and range)
70 ± 8.2 (53–88)
Number of exacerbation last year
0.8 (0–5)
BMI (kg/m2)
28.1 (20.3–49.4)
Lung function
FEV1% pred.
53.9 (27.0–95.6)
FEV1/FVC
46.4 (25.3–67.2)
Co-morbidities
Cardiovascular diseases
25 (67.6 %)
Respiratory failure depression
2 (5.4 %)
Diabetes
4 (10.8 %)
Adrenal tumor
1 (2.7 %)
Obstructive sleep apnea
1 (2.7 %)
Lung cancer
1 (2.7 %)
Therapy of patients
Oxygen
7 (18.9 %)
Inhaled glucocorticosteroids
15 (40.5 %)
Type of prosthodontic appliance
Complete acrylic denture
23 (62.2 %)
Partial acrylic denture
9 (24.3 %)
Partial framework denture
5 (13.5 %)
Clinical and laboratory studies included initial symptoms, interview about pain, burning sensation, and feeling dryness in the mouth. Intraoral examination was conducted by one of the authors. We examined the oral mucosa membranes and the hygienic condition of prosthetic appliances. Additionally, microbiological examinations included a direct swab from the surface of the dentures. The hygiene of acrylic mouth dentures was assessed using a modified Ambjørnsen et al. (1982) method for plaque evaluation in five areas of the denture: in three areas of the denture surface and two of the working side surfaces. Hygienic level was evaluated by the following criteria: no discernible denture plaque on visual examination or scrubbing with a dental instrument – good hygiene (0), moderate plaque covering part of the denture visible at examination – insufficient hygiene (1), and denture covered with visible plaque -poor hygiene (2). Denture stomatitis was diagnosed and classified according to modified Newton classification. Class 0 included patients with no inflammatory changes in oral mucosa, who complained of subjective syndroms, Class 1 included patients with pinpoints of surface inflammation on an unaltered mucosal membrane, Class 2 included patients with widespread inflammations under the denture, and Class 3 included cases with mucosal hyperplasia close to the denture.
Direct swabs were collected from acrylic denture surfaces and used for microbiological tests. The sample was immediately transported in Transwab containers to the Department of Medical Microbiology of Warsaw Medical University. Specimens were inoculated on liquid and gel medium, using Columbia agar with 5 % sheep blood and Chapman and MacConkey agar (bioMerieux, Marcy-l’Etoile, France). Plates were incubated for 48 h at 37 °C in an aerobic condition. Bacteriological identification was performed according to morphological and biochemical characteristics, using the bioMeriéux tests (Marcy-l’Etoile, France). Mycological inoculation was performed on the Sabouraud agar, where the yeast-like fungi were incubated at 30 °C for 48 h in the presence of oxygen. CHROMagar Candida selective medium (BioRad, Hercules, CA) and the assimilative ID32 C strip (bioMeriéux; Marcy-l’Etoile, France) were used for fungal growth analysis and identification. All microbiological sampling was conducted by a single examiner. Data were analyzed with a t-test with a statistical significance defined as p < 0.05.
3 Results
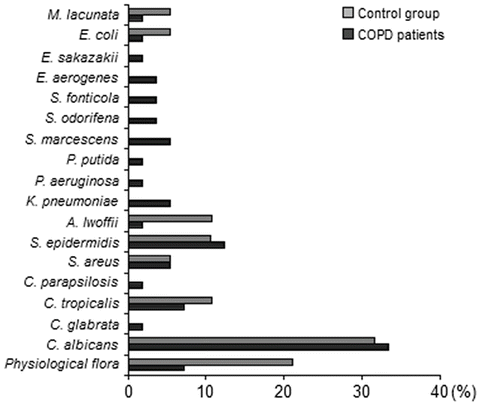
Aerobic bacteria were isolated from 36 COPD patients and 14 healthy denture users. Thirty-four of the thirty-seven swab samples from patients with COPD (91.9 %) yielded a positive culture of one or more potentially pathogenic microorganisms. The detection rates of aerobic organisms of denture plaques are summarized in Fig. 1. Candida spp. (43.9 %) and S. epidermidis (12.3 %) predominated in COPD patients and yeasts-like fungi (42.0 %) and S. epidermidis (10.5 %) in controls. A variety of bacteria potentially pathogenic for the respiratory system colonized the denture plaque in COPD patients. Among the isolates there were: S. aureus (5.3 %), K. pneumoniae (5.3 %), S. marcescens (5.3 %), Enterobacteriaceae spp. (7.0 %), and P. aeruginosa (1.8 %). In controls there were cases of growth of A. lwoffii (10.5 %), E. coli (5.3 %), and M. lacunata (5.3 %).