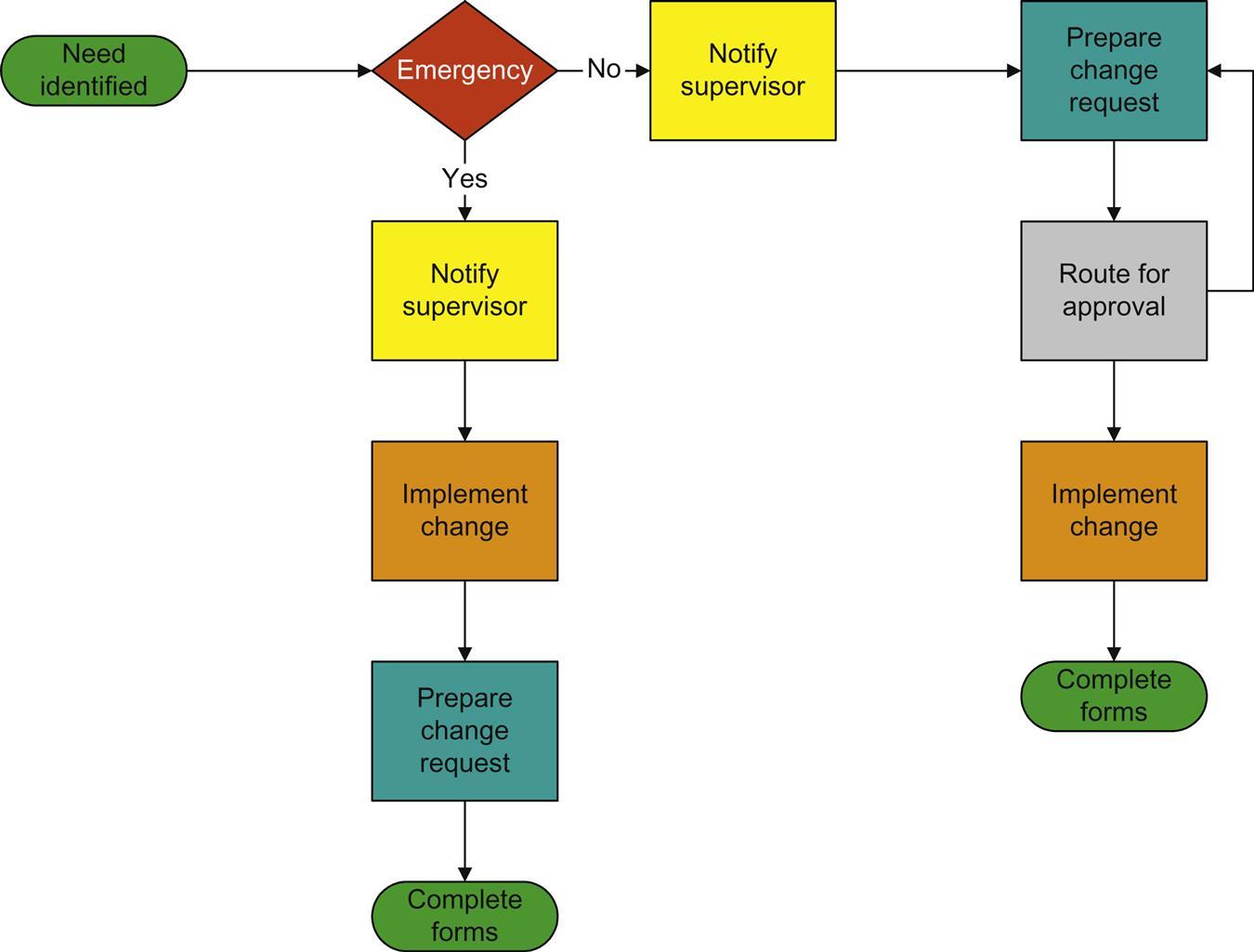
A major change requires that the FDA be notified, and approval obtained, prior to implementing the change. This type of change occurs when a piece of process equipment is changed (eg, changing blender types or designs, change of process parameters replacement of a “ribbon” blender with a “V” blender). Major changes are also those changes that may occur due to a change in the vendor for the active ingredient (API). The reason for this is that changing the API vendor or one type of process equipment for another, even if the function and capacity are the same, could result in a change in a Critical Quality Attribute (CQA) of the product; thus, jeopardizing the compliant state of the entire process.
A minor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree