9 1. The epidemiology of squamous oesophageal carcinoma is the most varied of any tumour, so that in certain geographical regions there is a very high incidence and in other areas it is rare. Squamous cell carcinoma of the oesophagus often shows multicentric occurrence in association with head and neck tumours, suggesting that environmental factors are important. Many such factors have been postulated, including smoking, alcohol, dietary nitrosamines, fungal contamination, pickled vegetables, hot tea, gruel, the habit of chewing betel leaf and areca nut and deficiency of certain vitamins and trace elements. A number of benign diseases predispose to its development, including corrosive alkali burns, achalasia, the Paterson-Kelly-Plummer-Vinson syndrome and a history of irradiation. Studies have suggested a link between enzymatic polymorphism related to alcohol degradation (ALDH2) and the development of squamous cell carcinoma of the oesophagus. Genetic predispositions include the autosomal dominant condition tylosis (Greek: tylos = callus) which results in hyperkeratosis of the skin, papillomas and eventually squamous carcinoma of the oesophagus. On the other hand, Barrett’s oesophagus is known to increase the risk of the development of adenocarcinoma: the incidence of adenocarcinoma of the lower oesophagus and gastric cardia is rapidly increasing in Western countries, although it is not clear if this increase is due to an increase in gastro-oesophageal reflux disease (GORD; gastro-oesophageal, hence GERD in the USA) and Barrett’s oesophagus. 2. The oesophagus runs from the neck through the thorax and into the abdomen. Apart from the cervical region, the oesophagus is divided into upper, middle and lower thirds, although the abdominal segment is sometimes considered separately. Approximately half of all squamous carcinomas develop in the middle third of the thoracic oesophagus. A much larger number of adenocarcinomas develop in the lower third in Western countries. 3. The tumour spreads circumferentially and longitudinally within the mucosa (intraepithelial spread) and in the submucosa and muscle layer, either continuously or separate from the main tumour (intramural metastasis). It may invade the trachea, bronchi, lungs, thoracic duct, recurrent laryngeal nerves, pericardium and aorta. Spread to lymph nodes, both local and distant, is common: detailed Japanese studies have shown that even upper-third carcinomas may be associated with nodal metastases inside the abdomen, while lower-third tumours involve the supraclavicular nodes in a significant proportion of patients. Haematogenous spread is relatively late, and affects the liver, lungs and bones. 4. Because of its insidious development, many patients present with advanced disease, complaining of dysphagia, weight loss, substernal or back pain, aspiration pneumonia or hoarseness. Supraclavicular lymph nodes may be palpable on presentation. The prognosis at this stage is very poor. 5. In Japan, a large number of asymptomatic squamous cell carcinomas are detected through endoscopic examinations at the time of routine health checks, and many of them are treated with endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Such local treatment is highly curative as long as the tumour invasion is limited to the epithelium and lamina propria mucosae. 6. Contrast radiography typically demonstrates a long, irregular stricture. However, early lesions produce only slight or even no mucosal irregularities on contrast barium swallow films. The diagnosis is confirmed by endoscopy with biopsy. The technique of Lugol’s iodine staining is useful as abnormal areas of mucosa (including carcinoma, oesophagitis, ectopic gastric mucosa and Barrett’s epithelium) remains unstained. Newer technologies of image enhanced endoscopy such as narrow band imaging (NBI) and flexible spectral imaging colour enhancement (FICE) may make early detection easier. Biopsy specimens should be taken from all suspicious lesions, where there is an irregularity or a colour change. Take a number of biopsy specimens and record the levels at which they are taken. Do not take many biopsy specimens from one lesion, particularly when the lesion may be suitable for endoscopic resection, because biopsies often result in submucosal fibrosis and may make later intervention difficult. 7. If the cytological brushings and biopsy specimens are reported to be normal but you suspect carcinoma, repeat the examination and go on repeating it until you are absolutely sure that you are not missing a cancer. Cancers located in the hypopharynx, cervical and the thoracic inlet oesophagus are the most difficult to detect. They are very often overlooked by otolaryngologists, radiologists and endoscopists. Particular effort should be taken at endoscopy to observe the hypopharynx, including both piriform sinuses, at the beginning of the examination and the oesophageal inlet on removal of the scope to avoid misdiagnosis. 8. Other valuable imaging modalities are computed tomography (CT), endoscopic ultrasonography and magnetic resonance imaging: these provide information on tumour size, invasion of contiguous structures and extent of lymph node involvement. Conventional endoscopic ultrasonography images the normal oesophageal wall as five to seven concentric layers; high-frequency ultrasound machines can visualize a nine-layered structure, allowing the depth of penetration to be estimated with greater accuracy. Ensure that CT scans include the upper abdomen and also the lower half of the neck, since enlarged lymph nodes that are not easily palpable may otherwise be missed. Conventional abdominal and cervical ultrasonography is also useful to detect abdominal and cervical nodal involvement, but remember that lymph node size is not diagnostic of metastasis: micrometastases are common and reactive hypertrophy of lymph nodes without metastasis is also frequent. FDG-PET is becoming routine for preoperative staging as it provides metabolic evidence of occult metastases, albeit with a significant incidence of both false negative and false positive results. Perform bronchoscopy with a flexible instrument in patients with middle- and upper-third advanced tumours to determine if there is invasion of the trachea or bronchi. 9. Preoperative staging should now be undertaken using the TNM classification of the International Union against Cancer (Table 9.1). 10. Carry out a careful assessment of the patient to exclude or confirm the presence of incidental disease, particularly that of the cardiovascular and respiratory systems. 11. Recent advances in the field of chemoradiotherapy (CRT) are rapidly changing the management of this disease. Although surgery remains the standard treatment for lesions where R0 resection is possible an increasing number of patients, particularly those with advanced disease, are treated with chemoradiation as the first mode of treatment. 12. As with many other fields of surgery, oesophagectomy has been influenced by the current trend towards minimally invasive surgery, and nowadays even Japanese-style extended radical oesophagectomy can be performed via Video-assisted thoracic surgery (VATS). Although the operation may not be truly minimally invasive because of the necessity for extended lymphadenectomy, the advantages of video-assisted surgery have been recognized. 1. Because oesophageal carcinoma is so often advanced at the time of diagnosis, some surgeons feel that treatment should be palliative, thus depriving patients who might be cured of the opportunity to benefit from a radical approach. Our experience of over 25 years of extensive lymph node dissection proves that even patients with ‘distant’ lymph node metastases regarded as M1 in the TNM system still have a chance of being cured by surgery. With the recent advance of non-surgical treatments, some T4 tumours have also become candidates for radical operation after neoadjuvant therapy. 2. Except in a few areas, oesophageal carcinoma is not a common disease. Unless patients are referred to a specialist centre, the clinicians who see them have relatively little experience on which to base management decisions. It has also been shown that surgeons who regularly perform oesophageal operations achieve lower mortality rates and better results than occasional operators. 3. There is general agreement, at least among surgeons, that radical resection is indicated in otherwise fit patients with tumours at the loco-regional stage (TNM Stage I-III, excluding T4b). However, there are different opinions about what constitutes radical resection. 4. There is more disagreement about the treatment options for patients with advanced tumours that involve adjacent structures (T4, particularly T4b) or have more extensive nodal involvement (N3 or with M1 lymph node metastasis). Some surgeons still apply radical surgery with curative intent together with vigorous multimodality treatments to these diseases as long as R0 resection is possible. Even when only palliative treatment is indicated, resection or bypass surgery remains an important option for achieving the best palliation, especially with regard to dysphagia. Radiotherapy (including concurrent CRT), chemotherapy, stenting, laser therapy and laser-activated photodynamic therapy are all claimed to be beneficial alone or in combination. In recent reports, concurrent CRT has been shown to be more effective than single-modality treatments. A surgeon working alone is unable to evaluate the various claims that are made. For this reason, work closely with your radiotherapy and oncology colleagues or refer your patients to a specialist centre. 5. Adequate resection of oesophageal carcinoma requires a tumour-free margin to avoid the development of recurrent malignant dysphagia. Because of the frequency of intraepithelial spread, intramural metastasis and multicentric tumours, precise preoperative diagnosis is of the utmost importance. Some surgeons send specimens from the upper and lower cut ends for frozen-section histology, but this does not entirely rule out the possibility of a ‘skip’ lesion remaining in the oesophageal remnant. 6. Do not make your decision without first discussing the possibilities and problems with the patient and, when appropriate, with the relatives. You must take into account the physical fitness and the psychological and philosophical attitudes of the patient. If you have obtained your patient’s confidence, you will usually be entrusted with making the decision. 1. Discuss the possibilities with the patient, since cooperation is essential to the success of major procedures. 2. Restore the dysphagic patient to a good nutritional state with soft oral foods and an elemental fluid diet, introducing a fine nasogastric tube into the stomach if necessary. Percutaneous endoscopic gastrostomy (PEG) is another option, particularly when neoadjuvant treatment requiring a longer time is planned. PEG is possible if a thin (transnasal) gastroscope can be introduced into the stomach, and it does not present an obstacle to future gastric pull-up. Parenteral intravenous alimentation is a useful option, although enteral feeding is more effective and preferred due to the concept of ‘immuno-nutrition’. 3. Oral hygiene is very important to prevent postoperative pneumonia. Preoperative consultation by a dentist should be performed routinely, and the patient should be trained in vigorous self-oral care. 4. Preoperative consultation by an otolaryngologist is also important. It might reveal unexpected recurrent laryngeal nerve palsy and meso- or hypopharyngeal cancer, which often occurs synchronously. Coincidental hypopharyngeal cancer is, however, more often detected by careful endoscopy. 5. Although enteral feeding can be re-started on the first or second postoperative day, set up a central venous line before surgery commences to secure reliable blood access. 6. Achieve the best possible cardiorespiratory state with physiotherapy, blood replacement and correction of serum protein and electrolytes. Smoking should be stopped as early as possible. Order 2–3 units of cross-matched blood. Collecting 800 ml of the patient’s own blood for autotransfusion is very useful to avoid allogenic blood transfusion. We also prepare cryoprecipitate from the patient’s own blood to apply it to the operative field as fibrin glue. 7. Ensure that the oesophagus, stomach and colon are empty. If the colon may be used for reconstruction, commence 1 or 2 days of low residue diet and administer oral bowel preparation the day before surgery (see Chapter 13). 8. Commence intravenous prophylactic antibiotic cover prior to commencing surgery: second- or third-generation cephalosporins have been used routinely. 9. Institute prophylaxis against deep venous thrombosis. Application of pneumatic cuffs to the lower extremities during and for several days after operation is most effective. Start continuous intravenous administration of heparin (100-150 units/kg/day) from immediately after the operation until the patient is mobile. 10. Undertake urethral catheterization and monitor hourly urine output during and after the operation. 11. Administration of a small dose of a corticosteroid (methylprednisolone, 250 mg) 2 hours prior to surgery is recommended by some authors to prevent the release of cytokines, which may precipitate ALI (acute lung injury) or ARDS (adult respiratory distress syndrome). 1. Our routine operation for carcinoma of the abdominal oesophagus and the cardia is resection of the lower thoracic oesophagus together with a cuff of the gastric cardia or entire stomach followed by jejunal interposition or Roux-en-Y reconstruction through the left thoracoabdominal approach (Fig. 9.1). Primary carcinoma of the lower thoracic oesophagus needs right thoracotomy for lymph node dissection. 2. You will nearly always find that the resection needs to go higher than you had planned. Technically, you can carry out anastomosis behind the carina through this approach. In doubtful cases, choose the right-sided approach, or bluntly dissect and resect the remaining upper thoracic oesophagus and then make an anastomosis in the neck between the cervical oesophagus and the gastric remnant or colon brought up either retrosternally or through the posterior mediastinum. 3. The VATS procedure is a combination of upper median laparotomy and left thoracoscopic operation assisted by the operator’s left hand inserted transhiatally (Fig. 9.1B). We call the conventional left thoraco-abdominal approach (LTA), and its VATS counterpart hand-assisted left thoracoscopic and abdominal approach (HLTA). 1. Place the anaesthetized, intubated patient on the right side, with the left shoulder rotated back against a support attached to the operating table, or use a self-retaining mat. 2. Open the left upper abdomen obliquely along the line of the sixth or seventh intercostal space, starting in the midline halfway between the umbilicus and the tip of the xiphisternum and extending to the left costal margin. The sixth intercostal space is more suitable when the lesion has substantial extension in the thoracic oesophagus, and mediastinal lymph node dissection is planned. Palpate the liver and the pelvis to rule out distant spread. Determine the fixity of the cardia and feel for extensive lymph node involvement that would make resection useless. Laparoscopic assessment of the abdominal cavity may demonstrate peritoneal dissemination and avoid unnecessary laparotomy. 3. If resection seems feasible, cut across the costal margin and elongate the skin incision toward the posterior axillary line along the planned intercostal space. Open the chest, cutting along the lower attachment of the intercostal muscles. Even in elderly patients with fixed ribs, sufficient separation of the intercostal muscles from the rib will allow adequate access. 4. Cut the diaphragm radially 10–15 cm towards the right crus. Even in very advanced case with direct tumour invasion to the crural muscle, you can achieve a safe surgical margin with circular resection of the muscle around the hiatus. It is not necessary to transect the diaphragm completely from its periphery to the part around the hiatus. If necessary, circumferential resection of the diaphragm around the hiatus can be added. 5. Insert a self-retaining rib retractor and gently open it in stages. 6. Stop all bleeding meticulously. 7. Anchor the edge of the incised diaphragm to the edge of the skin incision so that the left lung will not prolapse and interfere with the operative field during the intra-abdominal procedure. 1. Determine the extent of spread to the gastric cardia and glands along the left gastric vessels and around the coeliac axis. 2. Even if the stomach and associated glands appear to be uninvolved remove the left gastric basin, including the root of the left gastric artery. If the cardia or upper stomach is widely involved, prefer total gastrectomy. 3. If radical resection is inappropriate because of the extended disease, be willing to carry out an adequate longitudinal resection in a fit patient with a resectable tumour. Treat unresectable lesions by bypass, taking the stomach, jejunum or colon well above the tumour and joining it to the oesophagus in the chest or the neck. In Britain, gastro-oesophageal anastomosis in the neck is associated with the name of Kenneth McKeown of Northallerton. For fixed, extensive, obstructing tumours in high-risk patients, dilatation and insertion of an expandable metallic stent is the treatment of choice. Tube jejunostomy is also recommended for supplementary nutrition. 1. Open the lesser sac, dissecting the greater omentum from the transverse colon and severing the avascular portion of the gastric lesser omentum. 2. Ligate and divide each artery and vein of the stomach at the root according to the planned resection procedure. 3. Divide the stomach (for proximal gastrectomy) or the duodenal bulb (for total gastrectomy) with a linear stapling device. 4. Remove the anchoring stitch from the diaphragm and pass a tape through the hiatus. Pull the tape down to get good exposure of the left thoracic cavity. 1. Gently free the lower lobe of the left lung, dividing the pulmonary ligament, taking care not to injure the pulmonary vein. 2. Elevate and pull forward the lower lobe of the lung to display the posterior mediastinum. Locate the lower thoracic aorta and incise the mediastinal pleura just anterior to it. Dissect the anterior surface of the aorta. Ligate and divide the proper oesophageal arteries, which are rather rarely found in the lower mediastinum. 3. Incise the mediastinal pleura just posterior to the pericardium. Gently mobilize the lower oesophagus, leaving the mediastinal adipose tissue on the oesophageal side and taking care not to accidentally injure the azygous vein and the thoracic duct on the left side. If necessary, excise the posterior pericardium and a portion of the right pleura. 4. Decide the level of oesophageal transection and do not mobilize too high above this point. Too-extensive mobilization (more than 5 cm) of the oesophagus may result in ischaemia of the oesophageal stump. 5. Gently grasp the oesophagus with Akiyama’s oesophageal clamp or a right-angled vascular clamp and transect the oesophagus below it. Remove the specimen for histological examination. Immediate frozen section examination should be carried out if the upper margin is uncertain. Fig. 9.2 Standard reconstructive methods after resection of lower oesophagus and stomach: (A) jejunal interposition; (B) Roux-en-Y reconstruction. (Modified with permission from Akiyama H 1990 Surgery for cancer of the esophagus. Williams & Wilkins, Baltimore.) 1. Plan the mesenteric incision line under transillumination to obtain the longest jejunal loop. The second or third jejunal artery is usually most suitable as a feeder. Cut the jejunum between two clamps and pull the distal end up retrocolically through the hiatus to the oesophageal stump. 2. Place a running purse-string suture in the oesophageal stump and insert the anvil of a 25 mm circular stapling device. Insert the circular stapler through the open jejunal end to construct an end-to-side (functionally end-to-end) oesophagojejunostomy. Close the open jejunal stump using a linear stapling device. Add seromuscular sutures to bury the staple line. Insert a nasogastric tube though the anastomosis. 3. If the distal stomach is preserved, bury the line of staples on the gastric remnant with seromuscular stitches. Cut the elevated jejunum 20 cm below the hiatus, while preserving the feeding vessels. Make an anastomosis between the distal end of the jejunal segment and the anterior wall of the gastric remnant. Then form another end-to-end jejunojejunal anastomosis below the mesocolon to complete the reconstruction. 4. Following a total gastrectomy, Roux-en-Y reconstruction is a preferable choice. 5. We routinely omit Heineke-Mikulicz pyloroplasty in spite of truncal vagotomy. Although this may result in early-stage gastric stasis, patients have fewer episodes of bile regurgitation on long-term follow-up. 6. Make sure there is no tension on the anastomoses, and no twisting or kinking of the bowel. 1. Make sure the intrathoracic anastomosis is perfectly fashioned and there is no evidence of tension or ischaemia. An intrathoracic leak is often fatal. 2. Has all the bleeding stopped? 3. Close all the defects of the mesentery and mesocolon in order to avoid internal herniation. Fix the organ that was elevated for reconstruction to the hiatus to prevent prolapse into the thoracic cavity. Do not tighten the hiatus unless it was widened during the tumour resection. If you perform hiatal narrowing, make sure that the hiatus is still loose afterwards. 1. Insert an underwater-seal drain into the left pleural cavity through a separate stab incision near the costophrenic angle in the posterior axillary line. 2. Close the diaphragm using a continuous interlocking suture. The last 4–5 cm are closed with interrupted sutures from the abdominal side. Leave them unknotted until the incised intercostal space has been approximated with two thick, absorbable threads through both ribs. 3. Close the abdominal wound in your usual manner. 4. Close the chest wound, including the external oblique muscle and serratus anterior muscle above and below. 1. The two-stage operation of Ivor Lewis is the classic method for dealing with mid-oesophageal carcinoma. Many surgeons still employ this as their standard procedure for all oesophageal carcinomas. Newer and more radical techniques include the extensive (three-field) lymph node dissection popularized by Japanese surgeons. 2. VATS (video-assisted thoracic surgery) oesophagectomy is growing in popularity, as it offers advantages related to magnified view and variable view-point. It is also advantageous in surgical training because all the members of the team share the same view of the operative field and the instructor feels more confident when supervising a trainee performing a critical part of the operation. 3. It is not safe to embark on any of these advanced techniques, whether open or thoracoscopic, after simply reading a description. However, all the advanced techniques were developed as the result of step-by-step revision of older methods, enabling surgeons to make their operations more radical. 4. The more limited procedure of transhiatal oesophagectomy is still employed, combined with adjuvant therapy, and is claimed to be equally effective by its advocates. 1. This operation is the source of other newer and more radical operations. Therefore, the essential points of the Ivor Lewis operation also apply to newer techniques. Only the general concept of the operation and a few specific points are presented here. 2. The first step is the abdominal operation, in which the whole stomach is mobilized for reconstruction leaving the right gastric and gastroepiploic vessels as feeding vessels. The second step is the thoracic operation, involving oesophageal resection and reconstruction using pulled-up stomach as an oesophageal substitute. An oesophagogastrostomy is made intrathoracically. This is performed with an end-to-end circular stapling device or side-to-side application of the linear stapling device and is rarely performed manually these days. 3. By careful positioning of the patient, it is possible for two surgical teams to work simultaneously, placing the upper part of the patient in the position for right thoracotomy, with the pelvis almost flat on the operating table. The position may thereafter be adjusted by tilting the table. 4. Carry out a full Kocher’s mobilization of the head of pancreas and duodenal loop, because oesophageal reconstruction is usually done after closure of the abdominal incision. 1. Japanese surgeons have been performing extensive lymph node dissection for about 25 years, and more recently the technique has been adopted by surgeons in North America and Europe. The operative mortality is satisfactorily low and the 5-year survival rate exceeds 50% for those who undergo a curative (R0) resection. These good results are often attributed to differences in the type of tumour, population screening procedures, the general health and body habitus of the patients and stage migration due to extensive lymph node dissection itself (the Will Rogers phenomenon), but there is no good evidence that such factors play a part. 2. Achieving negative surgical margins for local control and extended lymphadenectomy for regional control are different but equally important targets of R0 resection. Skinner has advocated ‘en bloc dissection’ as a variation of such radical surgery. The essential concept of his operation is wide resection of tissues surrounding the oesophageal tumour, including mediastinal pleura, azygous vein, thoracic duct and pericardium for tumours with infiltrative growth beyond the adventitia. These days, such tumours are usually considered candidates for neoadjuvant chemoradiation. 3. The concept of extensive lymph node dissection was developed following investigation into the extent of lymphatic tumour spread, and made possible by careful attention to detail in planning and performing the operation. We do not take an inflexible attitude towards the extent of either oesophageal or lymph node excision, but try to match the operation to the site and extent of tumour growth and to the likelihood of invasion and spread to the lymph nodes. 4. Reliable preoperative assessment of the tumour by endoscopy, conventional ultrasound of the neck and abdomen, endoscopic ultrasound and CT is mandatory. 5. Although this preoperative assessment is helpful in detecting spread, it does not reveal micrometastases, so radical dissection must encompass what appears to be normal tissue. 6. Modern imaging techniques have made an initial abdominal exploration unnecessary in most patients. If uncertainty exists concerning resectability and curativity in the abdomen, start with the abdominal procedure. In such cases, the cervical and abdominal parts of the operation can be completed first (reconstruction-first approach). The order of mediastinal dissection and reconstruction is interchangeable so this is not a major problem. 7. Video-assisted radical oesophagectomy is now a standard procedure, particularly when the patient has no history of radio- or chemoradiotherapy. It is performed with the patient in a left lateral recumbent position similar to conventional open oesophagectomy. Because the essential operative technique of the two approaches is similar, details of the VATS procedure are appended to descriptions of the open procedure in the following paragraphs as necessary. Some surgeons prefer to perform VATS oesophagectomy in a prone position under positive pressure pneumothorax. Surgeons learning VATS oesophagectomy as part of a new team may find this approach easier, as it is almost ‘solo-surgery’ and no skilled assistant is necessary. However, for an experienced team the left lateral position has the advantage because the operation is performed within a view familiar to them. 1. Place the anaesthetized, intubated patient on the left side using a self-retaining mat. 2. Make a right anterolateral thoracotomy incision in the fourth intercostal space preserving the right latissimus dorsi muscle (Fig. 9.3). Fig. 9.3 (A) Skin incisions for the oesophagectomy with three-field lymphadenectomy. Thoracic incision is first made in left lateral recumbent position along the 4th intercostal space. The cervical and abdominal incisions are then made in supine position simultaneously by two teams. (B) Skin incisions for the video-assisted oesophagectomy with three-field lymphadenectomy. A minithoracotomy of 5 cm is placed along the 4th intercostal space. The numbers on the chest wall indicate the levels of intercostal spaces for each port for thoracoscopic surgery. A minilaparotomy of 7–9 cm and two port wounds are made for hand-assisted laparoscopic surgery. (Modified with permission from Akiyama H 1990 Surgery for cancer of the esophagus. Williams & Wilkins, Baltimore.)
Oesophageal cancer
INTRODUCTION
Appraise
Prepare
RESECTION OF CARCINOMA OF THE LOWER OESOPHAGUS (LEFT THORACO-ABDOMINAL APPROACH)
Appraise
Access
Assess
Resect
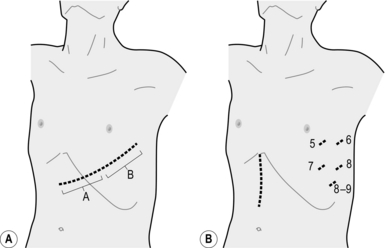
Abdominal procedure
Thoracic procedure
Unite (Fig. 9.2)
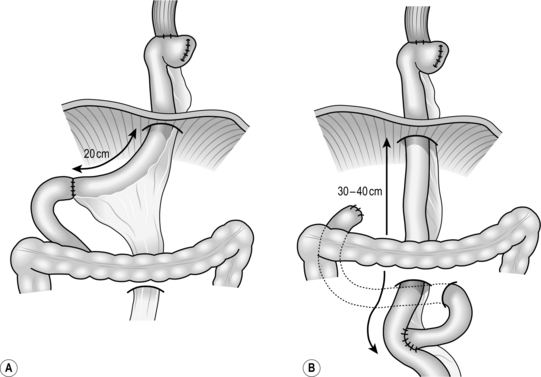
Checklist
Closure
OPERATIONS FOR THORACIC OESOPHAGEAL CARCINOMA
INTRODUCTION
IVOR LEWIS RESECTION FOR MID-OESOPHAGEAL CARCINOMA
Appraise
RADICAL CURATIVE SURGERY: EXTENSIVE LYMPH NODE DISSECTION (AKIYAMA TECHNIQUE)
Appraise
THORACIC PROCEDURE
Access
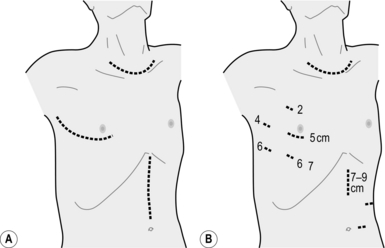
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree