(1)
Department of Pathology, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands
Keywords
JawNeoplasmsOdontogenic tumors5.1 Introduction
Mixed odontogenic tumors are composed of both epithelial and mesenchymal tissues. These tumors recapitulate proliferation and differentiation as seen in the developing teeth which often includes the deposition of the dental hard tissues – enamel and dentin, sometimes even leading to the development of multiple tiny teeth. Lesions with an identical histology can show either a neoplastic as well as an hamartomatous behavior [1].
5.2 Calcifying Cystic Odontogenic Tumor
The calcifying cystic odontogenic tumor forms part of a spectrum of lesions that in the past have been taken together under the designation calcifying odontogenic cyst and that have in common an ameloblastoma-like epithelial component with intraepithelial ghost cells to which other tissue components may be added [1].
Calcifying cystic odontogenic tumors occur both in maxilla and in the mandible in equal proportions. They cause bony expansion and radiographically, they manifest a lucent appearance with variable amounts of radiopacities (Fig. 5.1).
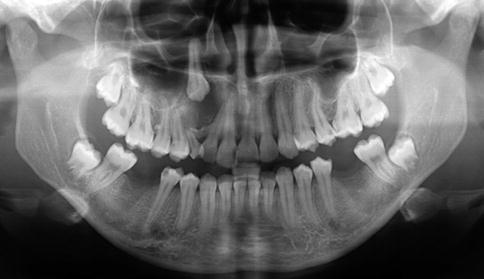

Fig. 5.1
Radiograph of calcifying cystic odontogenic tumor. A radiolucency surrounds the crown of the impacted right upper canine and has caused divergence of the roots of the erupted teeth in the same area
Histologically, the most simple appearance of a calcifying cystic odontogenic tumor is a cavity with a fibrous wall and an epithelial lining showing a basal layer of palisading columnar cells and a loose, edematous spinal layer similar as seen in unicystic ameloblastoma but with, different from the latter, also intraepithelial eosinophilic ghost cells (Fig. 5.2). These ghost cells may cluster to form large masses that occasionally herniate through the basal lamina to reach the underlying stroma, where they can act as foreign material and evoke a giant cell reaction (Fig. 5.3). Homogenous eosinophilic material, resembling dentin, may be found in varying amounts in the fibrous stroma adjacent to the basal epithelial cells, and this dentin and the ghost cells may form mixed aggregates (Fig. 5.4), a feature better appreciated in histochemical stains for collagen than in routine hematoxylin and eosin staining (Fig. 5.5).
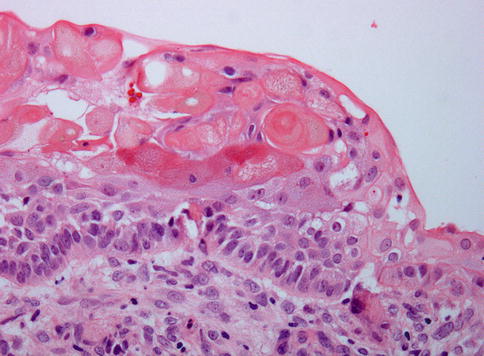
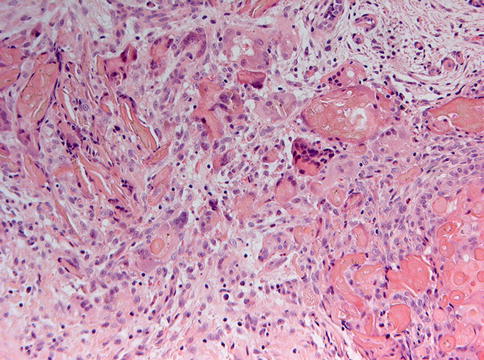
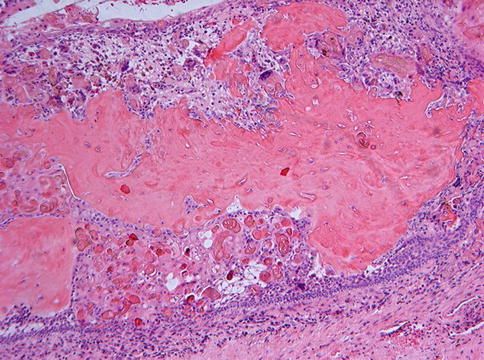
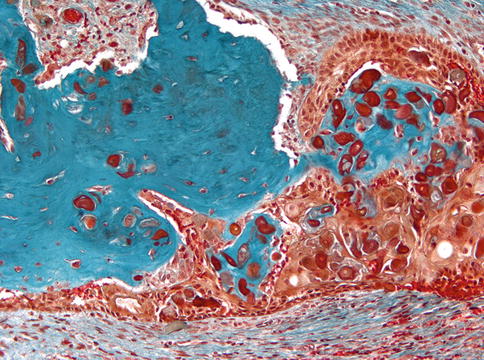

Fig. 5.2
Calcifying cystic odontogenic tumor. Cyst lining composed of epithelium showing basal palisading and loose superficial layers thus resembling ameloblastoma but with aggregates of intraepithelial ghost cells that make the distinction between both lesions

Fig. 5.3
Foreign body giant cell reaction occurs when the aggregates of ghost cells perforate through the basement membrane to make contact with the underlying stromal tissue

Fig. 5.4
Quite often, the ghost cells are surrounded by a matrix of homogeneous eosinophilic material considered to represent dysplastic dentin

Fig. 5.5
The distinction between dysplastic dentin and ghost cells is better appreciated in stains for collagen as with this Masson trichrome staining
This basic morphology may be modified by the addition of other components, which has led to the identification of different subtypes – the proliferative calcifying odontogenic cyst, showing multiple intramural daughter cysts with an epithelial lining similar to the main cyst cavity, and the solid (neoplastic) calcifying odontogenic cyst (differently named as: dentinogenic ghost cell tumor, epithelial odontogenic ghost cell tumor, calcifying ghost cell odontogenic tumor, and cystic calcifying odontogenic tumor) [2, 3].
To reduce this number of diagnostic labels to an acceptable level, the most recent WHO classification [1] has proposed the diagnostic designations calcifying cystic odontogenic tumor and dentinogenic ghost cell tumor to discern between the cystic and the solid variants.
The calcifying cystic odontogenic tumor/dentinogenic ghost cell tumor may cause considerable differential diagnostic problems due to morphological overlap with several other odontogenic tumors. In principle, a calcifying cystic odontogenic tumor is defined by three different features: (1) an epithelial component with the morphology of an ameloblastoma, (2) the presence of ghost cells either intraepithelially, stromal or both, and (3) juxtaepithelial collagenous material considered to be dysplastic dentin. The first problem is that ghost cells are not confined to calcifying cystic odontogenic tumor but may also occur in other odontogenic lesions, especially in odontomas (see below) and it is a matter of preference whether those should be classified as calcifying cystic odontogenic tumor with associated odontoma or as odontoma with additional features of a calcifying cystic odontogenic tumor [4]. The second point is that very rarely, lesions with the morphology of an ameloblastoma may nevertheless show abundant deposition of dysplastic dentin (Fig. 5.6), but lack any ghost cells. This item already has been discussed in Chap. 3 when referring to lesions provisionally labelled as adenoid ameloblastoma with dentinoid [5, 6].
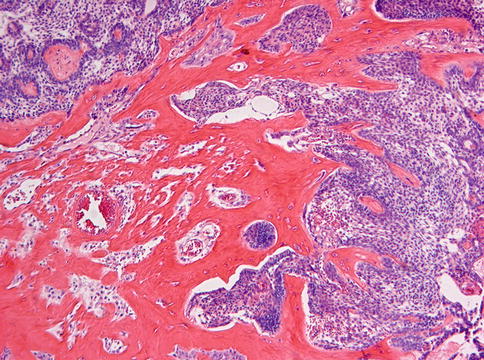

Fig. 5.6
Abundant formation of dysplastic dentin in relationship with an epithelial component showing the appearance of an ameloblastoma but without any ghost cells. These lesions could be classified as ameloblastoma with dentinoid or as dentinogenic ghost cell tumor with loss of the ghost cell component. This case showed no recurrence during a follow up time of 20 years after simple enucleation which argues against ameloblastoma as diagnosis
Furthermore, the calcifying cystic odontogenic tumor morphologically mimics craniopharyngioma to such an extent that the lesion may contain dental hard tissues to be interpreted as odontomas, but here, the distinction is easily made by the clinical presentation; the former being a jaw lesion and the craniopharyngioma occurring intracranially (Fig. 5.7) [7].
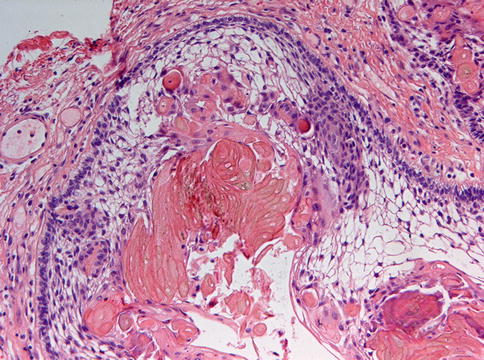

Fig. 5.7
Craniopharyngioma is also characterized by ameloblastomatous epithelium with ghost cell aggregates but with an entirely different site of occurrence
Treatment of the calcifying cystic odontogenic tumor consists of enucleation. Recurrences have been documented for both the cystic and the solid variant [4, 8]. Probably, these recurrences are due to the ameloblastomatous component. Therefore, in cases in which a calcifying cystic odontogenic tumor also shows features of an ameloblastoma, this latter component should probably dictate the most appropriate therapy, which consists of surgical excision including a margin of healthy tissue, but the issue how to treat this group of lesions cannot yet be considered to be completely settled, further work needed to define more precisely their biologic behavior [9].
5.3 Ameloblastic Fibroma
Ameloblastic fibroma is a neoplasm displaying soft tissues similar to those found in the immature tooth germ without any hard tissue component. Recently, it has been proposed that there are 2 variants of this lesion, lesions in patients aged above 22 years probably being true neoplasms, while those in younger patients may be either true neoplasms or odontomas in early stages of development [10]. So, this disease represents one of the examples of the ambiguous nature of the odontogenic tumors that recapitulate tooth formation; are they stages in the development of an hamartomatous lesion ending in a conglomerate of dentin and enamel or do they possess a genuine neoplastic potential, a problem having been discussed since decades [11] and unlikely to be solved satisfactorily.
Ameloblastic fibromas are rare [12]; they present as painless swelling of the posterior mandible or are discovered due to disturbances in tooth eruption. Radiographically, the tumor appears as a well-demarcated expansile radiolucency, often in connection with a malpositioned tooth.
Ameloblastic fibromas are composed of an admixture of epithelial and a mesenchymal elements. The epithelial part consists of branching and anastomosing epithelial strands that form knots of varying size. These knots have a peripheral rim of columnar cells that embraces a loosely arranged spindle-shaped epithelium (Fig. 5.8). The epithelial strands lie in a immature, myxoid cell-rich, mesenchyme (Figs. 5.9 and 5.10). The amount of epithelium may vary among different cases and regionally within an individual case. There is no formation of dental hard tissues and mitotic figures, either in epithelium and mesenchyme, are extremely rare; when easily found, they should rise concern about the benign nature of the case.
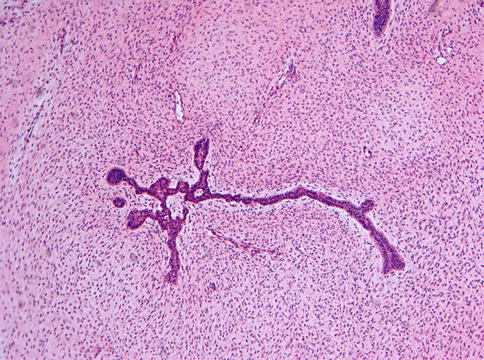
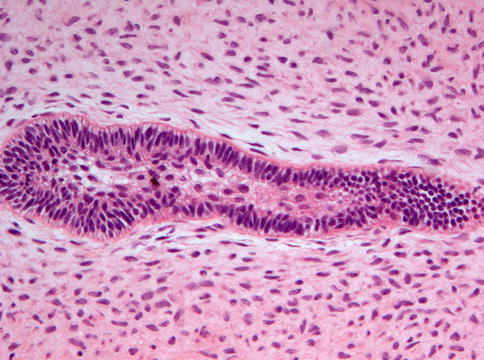
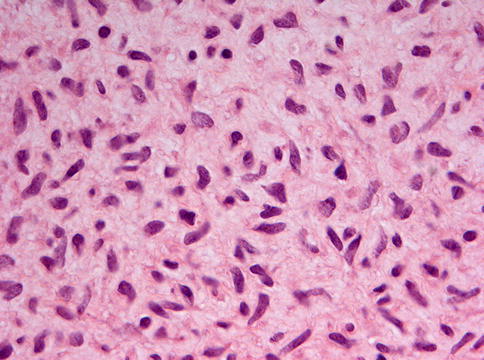

Fig. 5.8
Low power view of ameloblastic fibroma to show the elongated and branching and budding epithelial strands lying in a loose fibromyxoid background

Fig. 5.9
In ameloblastic fibroma, the epithelial component resembles ameloblastoma but the fibrocellular background makes the difference between both

Fig. 5.10
High power view of the stromal component of ameloblastic fibroma. Cells have plump and vesicular nuclei
Ameloblastic fibroma may contain granular cells: whether these lesions should be called granular cell ameloblastic fibroma or granular cell odontogenic fibroma is still controversial [13]. This issue already has been mentioned under the heading odontogenic fibroma in Chap. 4.
In cases with abundant epithelium, the main differential diagnostic issue is the distinction from follicular type ameloblastoma, discussed in Chap. 3. Ameloblastoma and follicular type ameloblastic fibroma share an identical epithelial component but the stromal component of ameloblastoma consists of mature fibrous connective tissue, whereas in ameloblastic fibroma, an immature, embryonic, cell-rich myxoid stroma is found. Sometimes however, myxomatous stromal degeneration in ameloblastoma may result in a superficial resemblance to the myxoid embryonic stroma present in ameloblastic fibroma but both can be distinguished by noting the differences in cellularity.
Whether the recently described primordial odontogenic tumor represents a genuine entity or just a morphological variation of ameloblastic fibroma has not yet been settled [14]. Cellular mesenchyme and columnar, inner enamel-like epithelium are features common for both but whereas in the latter the epithelial component is scattered within the mesenchyme, in the former it is located at the periphery, forming an external epithelial covering that may form invaginating strands (Figs. 5.11 and 5.12).
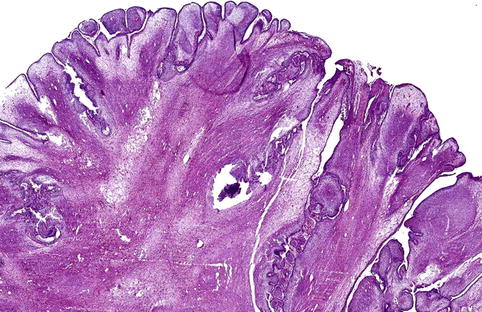
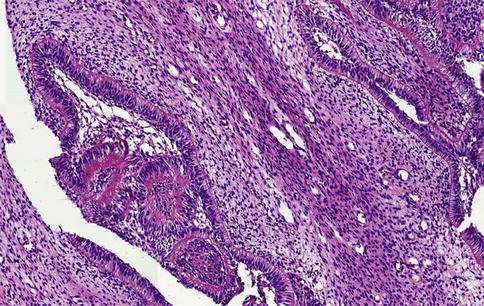

Fig. 5.11
Low power view of the lesion provisionally labeled primordial odontogenic tumor. Fibromyxoid tissue is covered with an outer layer of invaginating columnar epithelium

Fig. 5.12
Primordial odontogenic tumor. At the interface of fibromyxoid stroma and invaginating columnar epithelium, deposition of a collagenous matrix may occur. One could speculate whether this represents a first step towards development of more mature tooth tissues such as dentin and enamel
Areas similar to ameloblastic fibroma may also be observed in the hyperplastic dental follicle [15]. In such cases, the radiographic appearance makes the distinction: a radiolucent rim surrounding an unerupted tooth in the case of a dental follicle and an expansive radiolucent jaw lesion in the case of an ameloblastic fibroma. Moreover, in dental follicles, the areas mimicking ameloblastic fibroma are only small foci in a more fibrous background, such a predominance of fibrous tissue not being seen in ameloblastic fibroma (Figs. 5.13 and 5.14).
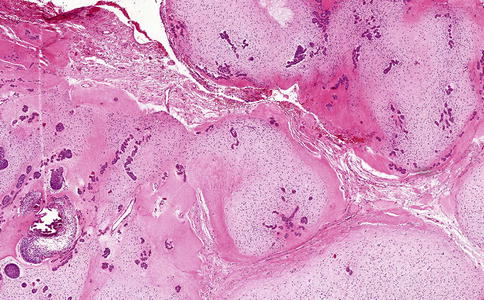

Fig. 5.13




In the fibrous tissue of a hyperplastic dental follicle, noduli resembling ameloblastic fibroma may be present. They should be considered tiny hamartomas of odontogenic tissue. Their nodular appearance allows distinction from ameloblastic fibroma that lacks any nodular architecture
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


