Fig. 9.1
Mild eyelid erythema and conjunctival congestion in acute keratopathy with mild SM exposure
A dose of 100–200 mg/m3/min can lead to moderate complaints involving eyelids, conjunctiva and cornea. Vesication often occurs in the corneal epithelium, most notably in the palpebral fissure. Almost similar symptoms mentioned in the mild category can be seen but with higher intensity. Besides, punctate erosions, chiefly on the interpalpebral fissure, arise on the corneal abrasion, infiltration, ulcer and eventually perforation 96, more often than not seen in middle and inferior cornea to the mild lesions, regression after 48 h and regeneration in 4–5 days, with full recovery procrastinated to 6 weeks comprise the concluding scenario in such cases (Fig. 9.2).
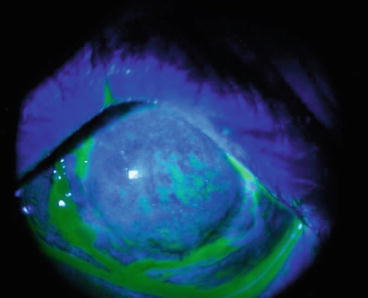

Fig. 9.2
Diffuse punctate keratopathy at the acute phase of keratopathy with moderate SM exposure
Lesions tend to intensify on exposure to in excess of 200 mg/m3/min, along with systemic toxicity involving skin, respiratory and GI systems (Solberg et al. 1997).
Ocular damage is admittedly more profound in the palpebral fissure and tempro-nasal zones owing to high exposure. Naso temporal limbi often show necrosis and whitening due to vascular involvement.
Eyelids may rarely adhere to the eye ball as toxicity often involves the exposed palpebral fissure. Low-grade iridiocyclitis minus synechia, cataract and full-thickness corneal injury and more often not, a temporary increase in intraocular pressure can be detected in exposure to doses greater than 400 mg/min/m3.
An “orange-peel” is a schematic description word phrase stromal edema as well major corneal epithelial defects, which remain stain-free on fluorescein application. In addition, possible nerve ending degeneration diminishes ocular protection, breaching corneal privilege in such condition. Epithelial erosions and ulcerations create an environment that makes the cornea prone to infections including Pseudomonas aeruginosa. Pseudomonas aeruginosa is the notorious super-infection culprit, urging therapeutic PK, or worse, evisceration. Deeper layers of cornea may also be affected in severe SM exposure when eyelids also ulcerate. This is seen in the form of severe ischemia and necrosis following early congestion and chemosis. Corneal edema resolution and as well as improving uveitis heralds recovery in 1–2 week (s). Intraocular bleeding may occur as a result of corneal neovascularization during recovery, leaving white opacities following degeneration. Superinfection may complicate the course of keratitis (Javadi et al. 2005; Solberg et al. 1997).
In severe toxicity, three scenarios can be described: the initial thorough resolution minus more inflammation, persistent complaints indicative of chronicity, late-onset lesions emerging years after. The first course often takes 2–6 weeks, with severe lesions disappearing but photophobia persisting. The patient will ultimately become symptom/sign-free. Throughout the chronic course, acute lesions recover, yet dry eye, photophobia and foreign body sensation perpetuates. There will also be sequelae namely limbal ischemia, corneal erosions and peripheral corneal neovascularization (Ashkenazi et al. 1991). Intrastromal exudation of plasma lipids and amyloid deposition can be seen in the latter scenario (Fig. 9.3). Deeper layers of the cornea are involved, creating descemetoceles, occasionally eventuating in perforation. What has to be borne in mind is that a “quiet eye” is not synonymous with resolution as inflammation may go on. The misleading on-going improvement in ocular conditions for weeks. Only to resurge even years (14–40) needs constant vigilance and attention.
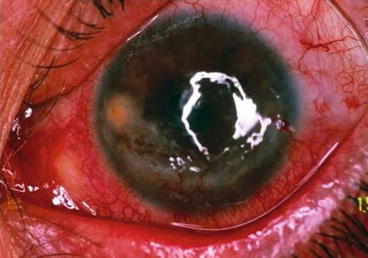

Fig. 9.3
Limbal ischemia, corneal abrasion and peripheral neovascularization as well as lipid keratopathy are sequels of acute phase mustard gas keratopathy
9.3.1.2 Chronic Phase
Irreversible, idiopathic corneal inflammation tends to occur in below 1 % of cases of acute intoxication with SM, often after a period of latency. This type of chronicity is often dubbed “MGK”. Having been initially detected in WWI veterans, the pathophysiology of MGK is not thoroughly recognized yet. Ever since, both experimental and human studies have been conducted to unearth the unknown pertaining to chronic ocular injury in such cases (Solberg et al. 1997).
Having investigated Iranian cases with delayed keratitis following a being asymptomatic for long revealed that chronicity is essentially distinct from acute circumstances, with MGK eventuating in permanent vision loss, either partial or total (United Nations Security Council 1984).
A number of theories have been put forward to account for the deterioration. An auto-immune process whereby corneal antigen attired by the offensive agent is destroyed, or a degenerative reaction which results in damage to cornea and limbus, toxic by-products and subsequent necrosis (confirmed by the presence of spindle-shaped keratocytes on confocal microscopy) are to name but a few (Saladi et al. 2006; Solberg et al. 1997; Javadi et al. 2005).
Delays in presentation can vary from 0.5 to 40 years in MGK, with slowly developing ocular discomfort as well as multisystem involvement. Ocular complaints resemble those seen in delayed form, yet they persist and progress to a full-blown inflammatory catastrophe. Limbus, conjunctiva and delayed involvements. The former is the most commonly affected while eyes may be involved asymmetrically (Balali-Mood et al. 2005).
Limbal lesions, identical to Mooren’s ulcer, present with infiltration and melting. Unless rendered avascular previously, vascular engorgement could also be seen, with tortuosity, segmental narrowing and sporadic dilated areas clear on observation. Narrowing in their extreme forms may make dilations appearing as blood pools (Etezad-Razavi et al. 2006).
Though seldom commencing intra-stromally, corneal problems more often than not presents with peripheral infiltration plus/minus thinning of the limb. As the condition recurs, the damage extends deeper into underlying layers, leaving crystalline deposits and giving cornea a silky appearance owing to progressive degeneration.
Neovascularization, causing irregularity and thinning on cornea, is an ominous premonition of later degenerative changes. Beside inflammation, melting recurs during relapse, forming descemetocele and causing perforation. Corneal sensation diminishes, intercellular epithelial attachments are impaired and basal tear secretion decreases, predisposing the eye to infections. Eye lid changes, similar to chronic blepharitis, include lid margin thickening and meibomian gland dysfunction.
In contrast, corneal lesions can be most detected in naso-temporal regions owing to higher exposure, likewise limbal vascularity are more affected in these areas, yet the account differs by nature as it is speculated that fewer number of stem cells makes the limbus prone.
Despite the report by Javadi et al. investigating the link between the course of the condition and climate dryness, the fact still remains to be unraveled via further investigation. What still perplexes researchers is the capricious nature of the disease, with erratic periodicity regarding exacerbation and relapse. Symptomaticity and ocular surface instability coincide with inflammation on an alternate basis (Javadi et al. 2005).
Grading of SM-delayed keratitis are as follows (Solberg et al. 1997).
Mild: deformed vessels on conjunctiva in the form of segmentation, tortuosity and telangiectasia minus corneal involvement (Fig. 9.4).
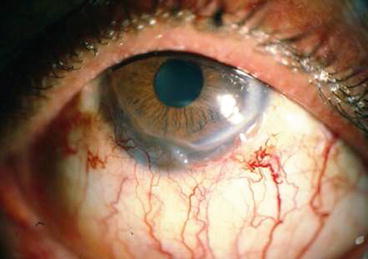
Fig. 9.4
Mild stage of delayed keratopathy following SM exposure
Moderate: conjunctivalisation: limbal ischemia and peripheral vascular invasion plus/minus corneal opacity (Fig. 9.5).
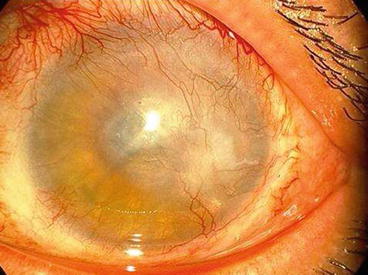
Fig. 9.5
Moderate stage of delayed keratopathy after SM exposure
Severe: conjunctival ischemia, corneal neovascularization, corneal thinning and melting and secondary degenerative changes (Fig. 9.6).
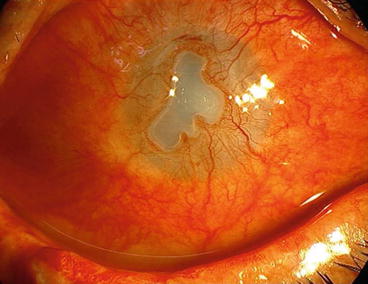
Fig. 9.6
Severe stage of delayed keratopathy due to SM exposure
Three mechanisms were proposed to account for delayed-keratitis presentations (Javadi et al. 2011):
1.
The on/going loss of stem-cells, initially incomplete and irregular but further progressing to conjunctivalisation, neovascularization and sustained epithelial erosions, limbal ischemia is also claimed to bring about neurotrophic and trophic changes namely thinning, descemetocele formation and perforation
2.
SM-induced vasculitis in the limbal vessels leads to sustained gradual ischemia mainly on the limb of the palpebral fissure.
3.
Lipoid and amyloid concentration, causes degenerative changes possibly in relation to chronic melting stromal inflammation and thinning.
There is no solid explanation to account for the exact effect of SM on limbal stem cells; nevertheless, higher grades of keratitis are known to be in a positive correlation with the severity of stem cell deficiency. Histopathological findings corroborate the assumption stressing the resilience of stem cells against the direct impact of the mustard gas owing to their low mitotic activity. Atypical conjunctival presentations namely telangiectatic, leaking, tortuous vascularity in peripheral cornea rather than completely vascularized pannus formation defy the account of goblet cells presence in relation with delayed MGK. Similarly, LSCD findings do not match corneal manifestations. A possibility is DNA alkylation in the stem cells, impairing epithelial regeneration. A secondary process, prolonged impaired innovation as a result of ischemia and diminished growth factors, is also claimed to have caused LSCD in the vicinity of stem cells. It is hypothesized that matrix metalloproteinase creates pathological conditions for stem cells in this respect. Stem cells deficiency may also explain cells pleomorphism as well as irregularity in boundaries on confocal microscopy. Another finding in the latter investigation regards the lack of sub basal nerve plexus, possibly owing to Bowman layer destruction and concurrence of sub epithelial fibrosis. Yet dry eye effect cannot be categorically denied. Typical wallerian degeneration as well as a severe process of the same nature inflicting nerves could be observed in animal experimentation on MGK. In line with this observation, sensation problems in the form of hypoesthesia/anesthesia were reported in cases where SM had led to neurotrophic keratitis (Javadi et al. 2007, 2011; Baradaran-Rafii et al. 2010).
SM exposure allegedly causes eye and skin inflammation in animal models. Yet, neither acute and delayed corneal injuries nor neovascularization did not seem to respond to anti-inflammatory agents as expected. As far as autoimmune process are concerned whereby altered corneal proteins (collagen-mustard compound) eventuates in keratitis, Naderi et al. failed to prove the efficacy of collagenase when applied to inhibit stromal collagen degradation. A plausible explanation in this respect will be that over-expression of collagenase can degrade normal stromal collagen, giving rise to pro-inflammatory cytokines. Hematoxylin-eosin staining on light microscopy reveals severe inflammation, stromal scarring and epithelial changes. A case in point is the disappearance of goblet cells, similar to what can be seen in dry eye condition. In a histopathological examination off 22 cases of chronic and delayed MGK, Javadi et al. encountered ulceration, thinning, diminishing epithelial cells, inflammation, vascularization and degeneration. Corneal buttons showed the deterioration of Bowman’s layer, epithelium, pannus formation neovascularization and stromal scarring. Necrosis was detected together with lipid and amyloid concentrations. Scrape cytology revealed conjunctival epithelial cell dysplasia in combatants formerly exposed to mustard gas. Aberrant pleomorphic keratocytes were reported on confocal microscopy of MGK cases, which is not normally seen in unoperated cases, perhaps owing to the mutagenicity as well as teratogenicity of the offensive agent (Javadi and Baradaran-Rafii 2009).
9.3.2 Posterior Segment
Electroretinographic (ERG) findings in an animal model showed no abnormality 6–7 weeks after exposure (Banin et al. 2003).
The study performed by authors (unpublished data) in survivors of Iran-Iraq war (1980–1988) about 20 years after exposure showed a general reduction of retinal photoreceptor function in chronic SM exposure. This effect involves both cone and rod photoreceptors in terms of amplitude and implicit time. There was also a general thinning of retina in macular area as evaluated by optical coherence tomography (OCT). These findings in ERG traces and OCT records of SM veterans show that SM intoxication may have late complications on neurologic tissues such as retina.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


