Overview
The overall burden of occupational cancer in Great Britain is currently around 8000 deaths and 14 000 cancer registrations per year. This represents 8% of all cancer deaths in men and about 2% in women
Asbestos contributes half of the deaths followed by silica, diesel engine exhaust, radon, work as a painter, mineral oils, shift work, environmental tobacco smoke in non-smokers, dioxins, radon and work as a welder
Work sectors at risk of occupational cancer include construction, painting and decorating, manufacturing, mining, quarrying, utilities and the service industry including personal and household services
Occupational cancer is a preventable disease and occupational health has an important role in primary prevention
Introduction
The first report of cancer caused by occupational exposure was in 1775 by Percival Pott, a British surgeon who described scrotal cancer in boy chimney sweeps. A century later, in 1895, Rehn, a German surgeon working in Frankfurt, treated a cluster of three cases of bladder cancer in workers at a local factory producing aniline dyestuffs from coal tar.
Occupational cancer is any malignancy wholly or partly caused by exposures at the workplace or in occupation. Such exposure may be due to a particular chemical (such as β-naphthylamine), a physical agent (such as ionizing radiation), a fibre like asbestos, a biological agent (such as hepatitis B virus) or an industrial process in which the specific carcinogen may elude precise definition (such as coke production).
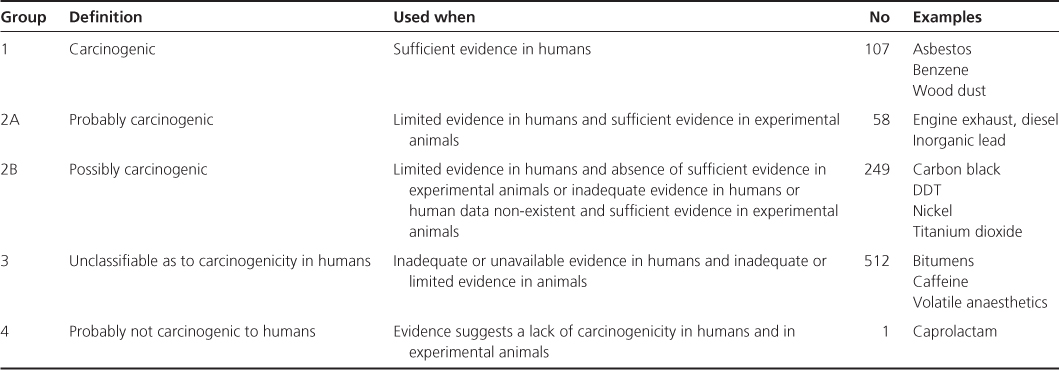
The International Agency for Research on Cancer (IARC) was set up to identify carcinogenic hazards to humans. Its role is to conduct and coordinate research into the causes of cancer. It maintains a series of monographs on the carcinogenic risks to humans posed by a variety of agents, mixtures and exposures. Since 1971 over 900 agents have been evaluated and the findings published in over 100 monographs. Over 400 agents have been identified as carcinogenic, probably carcinogenic or possibly carcinogenic to humans (Table 13.1).
Table 13.1 IARC classification
Full estimates of the current burden of occupational cancer from a consideration of all 24 cancer sites for which IARC has classified as definite or probable human carcinogens are gradually being published. Emerging findings suggest that the overall burden of occupational cancer in Great Britain is currently around 8000 deaths and 14 000 cancer registrations per year. This represents 8% of all cancer deaths in men and about 2% in women. The World Health Organization estimate that worldwide every year 200 000 die from cancer related to exposures in their workplace (Figure 13.1), and although historically the burden of occupational cancer has fallen mainly in developed industrialized countries, there is increasing concern for the potential growth of occupational cancer elsewhere as industry transfers around the world. This might be the relocation of unsafe processes such as asbestos manufacture or the transfer of old manufacturing equipment. In developing countries industry can be geographically dispersed or fragmented into small workshops where control measures are less likely to be used and less amenable to enforcement agencies or effective legislation, if available. Exposures to silica in small-scale mining, amines in local dye works and asbestos used in construction are good examples of situations where exposures are likely to occur. The concern therefore is that in years to come, the developing world could see a significant increase in the burden of occupational cancer, similar to or even exceeding that seen by the developed world during the twentieth century.
Mechanism
Cancer is a genetic disorder of somatic cells and can be triggered by the genotoxic action of carcinogens. Genetic aberrations can be found in the majority of human cancers. Oncogenes are normally suppressed in mature cells by regulating genes. DNA strand breaks, for instance due to ionizing radiation or chemical carcinogenesis with aberrant repair, can lead to loss of regulation. The activated oncogene uncouples the usual cell loss/gain equilibrium in favour of cell multiplication leading to an increase in cell numbers and ultimately appearance of a clinical tumour. Tumour suppressor genes exist in pairs within the cell and both must be inactivated for a tumour to develop. The p53 gene is the best known tumour suppressor (‘the guardian of the genome’) and is found to be mutated in the majority of sporadic cancers. Several environmental and occupational carcinogens are linked to p53 mutations such as UV light and skin cancer, tobacco and oral cancer. Other factors linked with p53 include alcohol, vinyl chloride and asbestos.
Tests for genotoxicity such as Ames and fluorescent in situ hybridization (FISH) are now well established. The Ames test is the most widely used procedure for assessing the mutagenicity of an agent, indicated by the number of bacterial colonies growing on a plate containing the toxic agent relative to those growing on a plate containing normal medium. FISH is used to assess chromosomal abnormalities.
Epigenetic carcinogens (also known as non-genotoxic or co-carcinogens) act more directly on the cell itself to cause abnormal cell proliferation and chromosomal aberrations that affect gene expression. These carcinogens have a threshold dose for carcinogenicity and it is possible to set exposure levels. For example, all workers involved in distilling β-naphthylamine eventually developed tumours of the urothelial tract, whereas only 4% of rubber mill workers who were exposed to β-naphthylamine contaminating an antioxidant (at 0.25%) used in making tyres and inner tubes developed bladder cancer over a 30-year follow up.
Polymorphisms are different responses to the same factor such as a drug. Slow acetylators who are heavy smokers are 1.5 times more likely to get bladder cancer if exposed to carcinogens. Certain polymorphisms increase the risk of mesothelioma 7.8 times. At the moment there are no readily available tests to determine susceptibility which are appropriate to workplace testing.
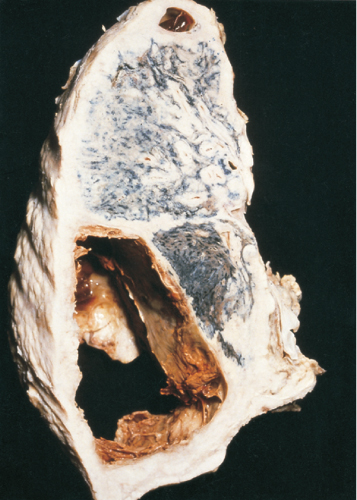
Occupationally related cancers are characterized by a long latent period (i.e. the time between first exposure to the causative agent and presentation of the tumour). This latency is not usually less than 10–15 years and can be much longer (40–50 years in the case of some asbestos-related mesotheliomas (Figure 13.2)), and, thus, presentation can be in retirement rather than while still at work. However, susceptibility to occupational carcinogens is greater when the exposure occurs at younger ages. An occupationally related tumour does not differ substantially, either pathologically or clinically, from its ‘naturally occurring’ counterpart.
Figure 13.2 Thick-walled mesothelioma of pleura with haemorrhagic cavitation in a former insulation worker.
Recognition and Diagnosis
For a group of workers, occupational cancer is evidenced by a clear excess of cancers over what would normally be expected. Some common malignancies that can be work related also have a well-recognized and predominant aetiology related to other agents, diet or lifestyle (for example, lung cancer from smoking). There are, however, some features which may help to distinguish occupational cancers from those not related to work (Box 13.1, Figure 13.3).
- History taking—Taking a patient’s occupational history is of paramount importance. It should be defined in detail and sequentially. For example, a holiday job in a factory that lasted only a few months could easily be overlooked, but it may have involved delagging a boiler or handling sacks of asbestos waste.
- Signal tumours—Several uncommon cancers are associated with particular occupations. Thus, an angiosarcoma of the liver may indicate past exposure to vinyl chloride monomer in the production of polyvinyl chloride although there have been no cases in workers exposed since 1969. A worldwide registry of all exposed workers exists maintained by the Association of European Plastics Manufacturers.
- Age—A younger age at presentation with cancer may suggest an occupational influence. For example, a tumour of the urothelial tract presenting in anyone under the age of 50 years should always arouse suspicion (Figure 13.4).
- Patients’ information—Patients may speak of a ‘cluster’ of cancer cases at work or may have worked in an industry or job for which a warning leaflet has been issued.
- detailed lifelong occupational history
- comparison with a check list of recognized causal associations
Confirmation of requisite exposure
- Search for additional clues: Shift to a younger age; Presence of signal tumours; Other cases and ‘clusters’; Long latency; Absence of anticipated aetiologies; Unusual histology or site such as angiosarcoma in vinyl chloride workers and renal pelvic tumours following exposure to amine dyes
Prevention and Management in the Workplace
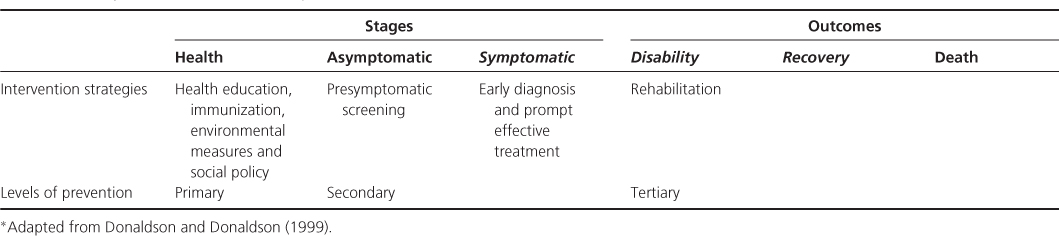
Primary prevention seeks to actually prevent the onset of a disease. Secondary prevention aims to halt the progression of a disease once it is established. Tertiary prevention is concerned with the rehabilitation of people with an established disease to minimize residual disabilities and complications or improve the quality of life if the disease itself cannot be cured (Table 13.2).
Table 13.2 Stages and outcome in cancer cycle
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





