Obesity
KEY CONCEPTS
![]() Two clinical measures of excess body fat, regardless of sex, are the body mass index (BMI) and the waist circumference (WC). BMI and WC provide a better assessment of total body fat than weight alone and are independent predictors of obesity-related disease risk.
Two clinical measures of excess body fat, regardless of sex, are the body mass index (BMI) and the waist circumference (WC). BMI and WC provide a better assessment of total body fat than weight alone and are independent predictors of obesity-related disease risk.
![]() Excessive central adiposity increases risk for development of type 2 diabetes, hypertension, and dyslipidemia.
Excessive central adiposity increases risk for development of type 2 diabetes, hypertension, and dyslipidemia.
![]() Weight loss of as little as 5% of total body weight can significantly improve blood pressure, lipid levels, and glucose tolerance in overweight and obese patients. Sustained, large weight losses (e.g., after bariatric surgery) are associated with long-term improvements in many of the complications associated with obesity and a lower risk of both myocardial infarction and death.
Weight loss of as little as 5% of total body weight can significantly improve blood pressure, lipid levels, and glucose tolerance in overweight and obese patients. Sustained, large weight losses (e.g., after bariatric surgery) are associated with long-term improvements in many of the complications associated with obesity and a lower risk of both myocardial infarction and death.
![]() Bariatric surgery may be considered in patients with extreme obesity with a BMI ≥ 40 kg/m2 or ≥ BMI ≥35 kg/m2 with significant comorbidities.
Bariatric surgery may be considered in patients with extreme obesity with a BMI ≥ 40 kg/m2 or ≥ BMI ≥35 kg/m2 with significant comorbidities.
![]() Pharmacotherapy may be considered in patients with a BMI ≥30 kg/m2 and/or a WC ≥40 inches (≥102 cm) for men or 35 inches (89 cm) for women, or BMI of 27 to 30 kg/m2 with concurrent risk factors if 6 months of diet, exercise, and behavioral modification fail to achieve weight loss.
Pharmacotherapy may be considered in patients with a BMI ≥30 kg/m2 and/or a WC ≥40 inches (≥102 cm) for men or 35 inches (89 cm) for women, or BMI of 27 to 30 kg/m2 with concurrent risk factors if 6 months of diet, exercise, and behavioral modification fail to achieve weight loss.
![]() Long-term pharmacotherapy with centrally acting appetite suppressants should be discontinued if weight loss of at least 5% is not achieved after 12 weeks of maximum-dose therapy.
Long-term pharmacotherapy with centrally acting appetite suppressants should be discontinued if weight loss of at least 5% is not achieved after 12 weeks of maximum-dose therapy.
![]() There is a high probability of weight regain when obesity pharmacotherapy is discontinued.
There is a high probability of weight regain when obesity pharmacotherapy is discontinued.
![]() The Food and Drug Administration does not regulate labeling of herbal and food supplement diet agents, and content is not guaranteed.
The Food and Drug Administration does not regulate labeling of herbal and food supplement diet agents, and content is not guaranteed.
INTRODUCTION
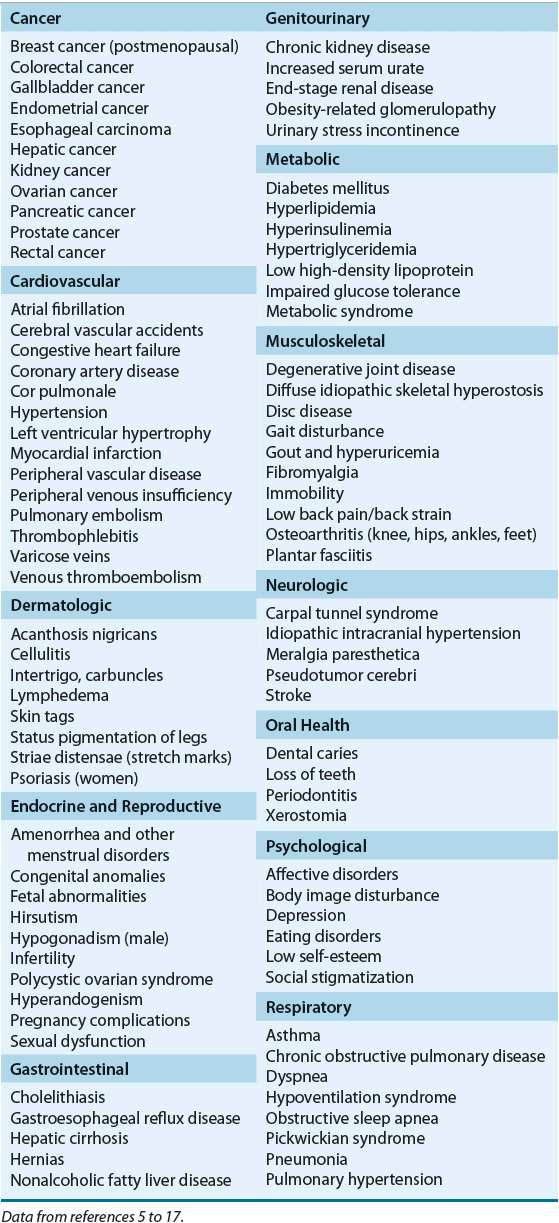
It is now estimated that more than 140 million or two of every three adults are overweight or obese in the United States.1 Additionally, the number of children and adolescents who are overweight has been increasing at an alarming rate in the last 40 years,2 with one of every three adolescents currently considered overweight or obese.3 Based on the national trend, this epidemic is projected to affect about 80% of the U.S. adults by 2020, and the prevalence of overweight among children is expected to double by 2030.4 The presence of obesity and overweight is associated with a significantly increased risk for the development of many diseases (Table 121-1),5–17 poorer outcomes of comorbid disease states, and increased healthcare costs. Prospective cohort studies show that overall mortality parallels increases in adiposity.18,19 The evidence is strongest for middle-aged adults. In older individuals, excess body weight and adiposity increase the risk of death, but the degree of impact diminishes with age.18,19 As of 2008, it was estimated that obesity accounts for 9.1% of total medical expenditures in the United States, and the cost of treating obesity-related illnesses in adults approached national health spending of $147 billion annually.20 National initiatives to reverse the obesity epidemic have been established through prevention strategies, consensus guidelines, and best practices.21–25 This chapter reviews the epidemiology, pathophysiology, and therapeutic approaches for the management of obesity. Although nonpharmacologic treatment modalities are discussed, the pharmacotherapy of obesity is highlighted, and the role of pharmacotherapy relative to the other therapeutic options is critically reviewed.
TABLE 121-1 Conditions More Prevalent Among Patients with Obesity
EPIDEMIOLOGY
Obesity in the United States has increased in prevalence since the 1960s. The National Health and Nutrition Examination Survey (NHANES) II data (1976–1980) estimated the prevalence of obesity among adults in the United States at 15%.2 During NHANES 1999 to 2000, the prevalence increased twofold to 30.9%,2 and by 2010 obesity affected 35.9% of the adult population,1 making the prevention of obesity a public health priority.23 This is further emphasized by the continued pursuit of safe and effective long-term therapies for obesity. Existing evidence consistently suggests that children who are overweight are at least twice as likely to remain overweight as adults compared with normal-weight children.26 Furthermore, overweight or obese children and adolescents have a higher risk of premature mortality and morbidity as adults.27 Therefore, childhood and early adulthood are critical intervention periods for prevention of obesity in the future. The prevalence of obesity varies by sex among racial and ethnic minorities within the United States.2 The highest prevalence is observed among non-Hispanic black women (58.5% obese and 17.8% with extreme obesity) compared with values of 38.8% and 7.4% for non-Hispanic black men, respectively.1 This gender disparity is also associated with the level of parental education. Young black women from the lowest educated families are at greater risk of obesity compared with young black men.28 The prevalence of obesity also increases with age, reaching a maximum by the eighth decade.2 After the age of 80 years, the prevalence falls progressively for both genders. Socioeconomic status clearly affects the prevalence of obesity among non-Hispanic white adults; a strong inverse association is observed among non-Hispanic white women from lower socioeconomic classes.2 Educational achievement, which is linked to socioeconomic status, is also correlated with the fraction of people who are overweight; the prevalence of overweight is greatest in those with less than a high school education.
ETIOLOGY
Obesity occurs when there is increased energy storage resulting from an imbalance between energy intake and energy expenditure over time. The specific etiology for this imbalance in the vast majority of individuals is multifactorial, with genetic and environmental factors contributing to various degrees. In a small minority of individuals, excess weight may be attributed to an underlying medical condition or an unintended effect of a medication.
Genetic Influences
Observational studies in humans and experimental studies in animal models have demonstrated the strong role of genetics in determining both obesity and distribution of body fat. In some individuals, genetic factors are the primary determinants of obesity, whereas in others, obesity may be caused primarily by environmental factors. The genetic contribution to the actual variance in body mass index (BMI) and body fat distribution is estimated to be up to 80%.29 The increase in the prevalence of obesity that has taken place in the United States over the past 40 years is without doubt the result of alterations in our environment that readily allow obesity-promoting genotypes to cause excessive adiposity.
The role of genetic influences in the development of obesity is an area of extensive research. A number of single-gene mutations producing extreme obesity have been identified, but such mutations are rare and account for an extremely small number of the total cases of obesity.30 Some common alleles—for instance, the rs9939609 obesity-risk allele in the FTO (fat mass and obesity associated) gene that is found in almost 70% of people—increases BMI by about 2 kg/m2.31 The total number and identity of contributing genes are still being determined, as is the means by which the many potential so-called “obesity” genes interact with each other and with the environment to produce the obese phenotype.
Environmental Factors
Many of the societal changes associated with economic development over the past 40 years have been implicated as potential causes for the increase in the prevalence of obesity. These include an abundant and easily accessible food supply and the material comforts of modern life in Western civilizations, which have contributed to a reduction in physical activity.32 Advances in technology and automation have resulted in more sedentary lifestyles during both work and leisure time for most individuals. At the same time, there has been a significant increase in the availability and portion size of high-fat foods, which are aggressively marketed and are often more convenient and less expensive than healthier alternatives. This modern environment has been described by some as “obesogenic” because it is likely to result in a state of positive energy balance in many individuals (Fig. 121-1).33 Obesity has also been reported more frequently among individuals within close social networks (e.g., siblings, spouses, and friends), with a person’s risk of becoming obese increasing significantly if a friend in his or her social network is obese.34 Finally, it should be noted that cultural factors, socioeconomic status, and religious beliefs may influence eating habits and body weights.
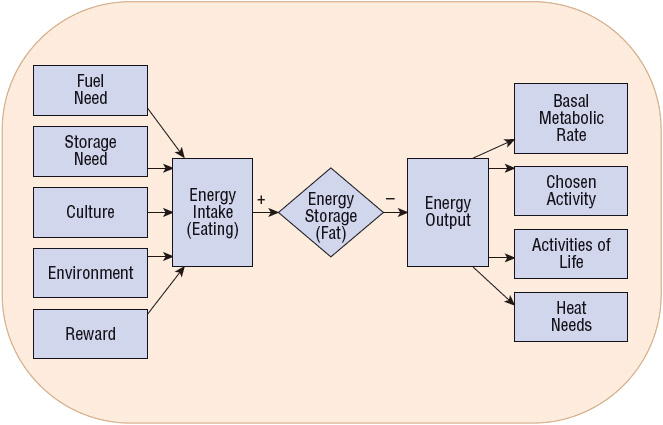
FIGURE 121-1 Net energy stores are determined by various inputs and outputs. Simply stated, obesity occurs when there is an imbalance between energy intake and expenditure.
Medical Conditions
Occasionally, patients present with obesity secondary to an identifiable medical condition. Conditions associated with weight gain include iatrogenic and idiopathic Cushing’s syndrome, growth hormone deficiency, insulinoma, leptin deficiency, and various psychiatric disorders, such as depression, binge-eating disorder, and schizophrenia. Hypothyroidism is often included in this list, but it mostly causes fluid retention (myxedema) and is generally not a cause of significant obesity. Genetic syndromes that have obesity as a major component are extremely rare and include Prader-Willi’s, WAGR (Wilms’ tumor, aniridia, genitourinary abnormalities or gonadoblastoma, and mental retardation), Simpson-Golabi-Behmel’s, Cohen’s, Bardet-Biedl’s, Carpenter’s, Börjeson’s, and Wilson-Turner’s syndromes. The clinician evaluating a patient for obesity needs to be aware of these potential conditions. The physical examination of obese patients always should include an assessment for secondary causes of obesity, including genetic syndromes.
Medications
An increasing number of medications are associated with unintended weight gain.35 These include several anticonvulsants (e.g., carbamazepine, gabapentin, pregabalin, and valproic acid), antidepressants (e.g., mirtazapine and tricyclic antidepressants), atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, and risperidone), conventional antipsychotics (e.g., haloperidol), and hormones (e.g., corticosteroids, insulin, and medroxyprogesterone). Although the pharmacologic mechanism responsible for weight gain is usually drug-specific, in most cases the precise mechanism is unknown.
PATHOPHYSIOLOGY
The pathophysiology of obesity involves numerous factors that regulate appetite, energy storage, and energy expenditure. Disturbance of these homeostatic functions results in an imbalance between energy intake and energy expenditure.
Appetite
Human appetite is a complex process that is the net result of many inputs within a neural network involving principally the hypothalamus, limbic system, brainstem, hippocampus, and elements of the cortex.36 Within this neural network, many neurotransmitters and neuropeptides have been identified that can stimulate or inhibit the brain’s appetite network and thereby affect total caloric intake.
Biogenic Amines
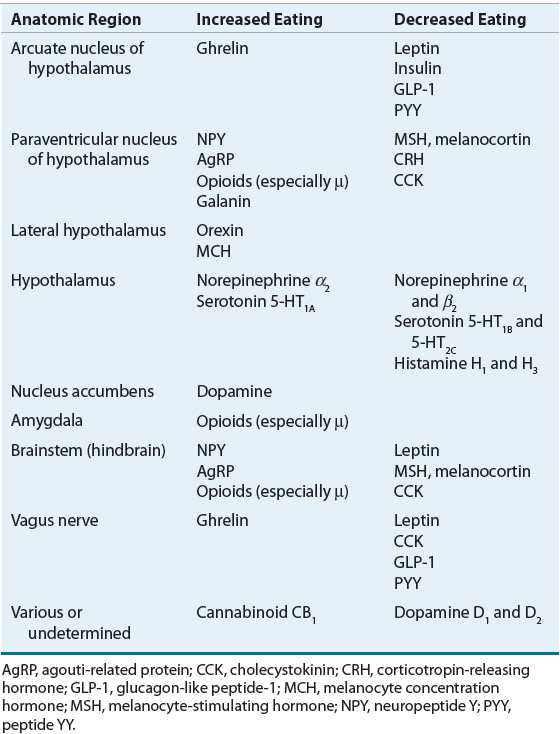
The first receptor systems found to alter food intake in animals and humans were the biogenic amines. These neurotransmitters are the foundation from which the most robust pharmacologic interventions for obesity have been developed. Serotonin, also known as 5-hydroxytryptamine (5-HT), and cells known to respond to 5-HT are found throughout the central nervous system (CNS) and the periphery. Currently, two major noradrenergic receptor subtypes are recognized (α and β), each with multiple subtypes. Histamine and dopamine also demonstrate multiple receptor subtypes, but their role in the regulation of human eating behaviors and food intake is less well documented. Direct stimulation of 5-HT1A and noradrenergic α2-receptors increases food intake; the opposite occurs with 5-HT2C and noradrenergic α1– or β2-receptor activation. Table 121-2 summarizes the major effects of direct receptor stimulation, inhibition, and changes in synaptic cleft amine concentrations on food intake.
Neuropeptides
Many neuropeptides that influence appetite exert their effects within the hypothalamus. Thus, in the last several years research has focused on the neural projection between parts of the hypothalamus and the arcuate nucleus with signals to the paraventricular nucleus. The key peptides in this projection are currently thought to be neuropeptide Y and α-melanocyte–stimulating hormone, which engages melanocortin receptors in the paraventricular nucleus. Neuropeptide Y is the most potent known stimulator of eating, and α-melanocyte–stimulating hormone action at the melanocortin 3 and 4 receptors is one of the crucial inhibitors of eating.36,37
The lateral hypothalamus has been referred to as the “hunger” center within the brain. The most prominent of these lateral hypothalamic peptides, orexin, increases food intake stimuli within the lateral hypothalamus.36 Another important neuropeptide stimulator of eating that principally originates in the lateral hypothalamus is melanocyte-concentrating hormone. Neurons in the lateral hypothalamus use orexin and melanocyte-concentrating hormone to communicate with other neurons throughout the brain and thereby affect a number of functions beyond appetite.36,37 Table 121-2 summarizes the major effects of various neuropeptides on food intake. Although hunger and satiety functions are thought to be primarily regulated by the hypothalamus, humans eat in response to a broad set of stimuli, including reward, pleasure, learning, and memory.
Peripheral Appetite–Related Signals to the Brain
Peripheral appetite signals also dramatically affect food intake. Leptin, a hormone that is secreted by adipose cells, acts on the arcuate nucleus of the hypothalamus and elsewhere in the brain to decrease appetite and increase energy expenditure.38,39 Studies conducted in leptin-deficient mice and humans revealed that exogenous leptin administration produced significant weight loss. However, recombinant leptin replacement therapy in obese humans who are not leptin deficient has not proved successful because obese humans appear to be leptin resistant. Figure 121-2 shows the peripheral link that leptin appears to provide in signaling the CNS about the status of fat cell mass. Leptin also has been found to regulate various functions outside the CNS, including insulin and glucocorticoid secretion, reproduction, and glucose transport within the small intestine.38
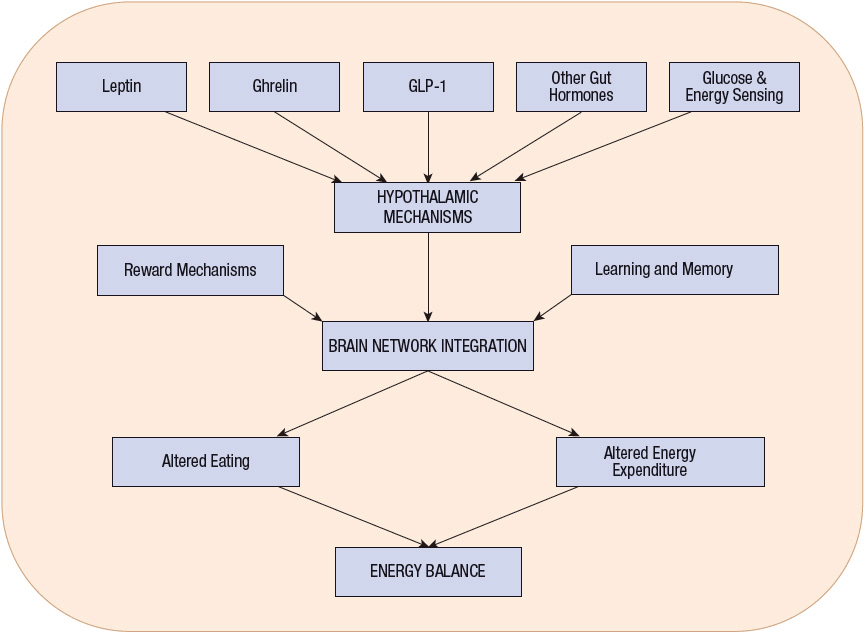
FIGURE 121-2 Intrinsic hypothalamic hunger and satiety mechanisms are modified by input from fat tissue via leptin, and from the gut via ghrelin, glucagon-like peptide-1 (GLP-1), and other hormones. Additional input is derived by direct sensing of prevailing glucose and other energy signals. The hypothalamus generates signals that are integrated within brain networks, which also receive additional signals. The brain network effects change in energy balance by modifying food intake and energy expenditure.
Other peripheral signals important to the brain’s processing of appetite include several gut hormones, notably those released by the intestine in response to passage of digesting food such as glucagon-related peptide-1, oxyntomodulin, and peptide YY.40 Each of these hormonal signals suppresses eating in animals and humans. Glucagon-related peptide-1 has other effects, most importantly as an incretin, which facilitates release of insulin by pancreatic β cells in response to meal-related glucose. Ghrelin, another important gut hormone that is released from the distal stomach and duodenum, stimulates appetite.
An understanding of the relationships among the brain, its many neurotransmitters and neuropeptides, environmental stimulation of brain activities, and other hormones is still evolving. Dysfunction in any of these factors can upset the homeostatic functions regulating energy balance. Exogenous manipulation of neural signals and associated peripheral hormones may provide future pharmacotherapeutic targets for obesity management.
Energy Balance
The net balance of energy ingested relative to energy expended by an individual over time determines the degree of obesity (see Fig. 121-1). An individual’s metabolic rate is the single largest determinant of energy expenditure. Resting energy expenditure (REE) is defined as the energy expended by a person at rest under conditions of thermal neutrality. Basal metabolic rate (BMR) is defined as the REE measured soon after awakening in the morning at least 12 hours after the last meal. Metabolic rate increases after eating based on the size and composition of the meal. It reaches a maximum approximately 1 hour after the meal is consumed and returns to basal levels 4 hours after the meal. This increase in metabolic rate is known as the thermogenic effect of food. The REE measures the energy costs of the wakeful state and may include the residual thermogenic effect of a previous meal; it is thus usually higher than the metabolic rate measured during quiet sleep. Physical activity is the other major factor that affects total energy expenditure and is the most variable component.
Peripheral Storage and Thermogenesis
There are two major types of adipose tissue, white and brown. The primary function of white adipose tissue is lipid manufacture, storage, and release. Brown adipose tissue, once believed to be found only in infants, is now recognized to exist in most adults.41 It is more commonly identified in lean than obese individuals, but its importance for human obesity remains unclear. Whereas lipid storage occurs in response to insulin, lipid release is seen during periods of calorie restriction. Brown adipose tissue is notable for its ability to dissipate energy via uncoupled mitochondrial respiration.42 Both white and brown adipose tissues are highly innervated by the sympathetic nervous system, and adrenergic stimulation via β-adrenergic receptors (β1, β2, and β3) is known to activate lipolysis in fat cells as well as increase energy expenditure in adipose tissue and skeletal muscle. Genetic polymorphisms have been identified in both the β2– and β3-receptor systems that are associated with obesity or excess weight gain.43 Thus, genetic susceptibility for excess weight status may in part be related to adrenergic dysfunction.
CLINICAL PRESENTATION
Although obesity is readily apparent, most obese patients seek healthcare only when obesity-associated comorbidities become problematic. A consistent and reproducible description of weight status is essential in the diagnosis and management of obesity. Evidence-based guidelines issued by many groups, notably the World Health Organization and the National Institutes of Health (NIH), have established a stratification of weight excess based on associated medical risks.44 The first increment of excess weight is termed overweight, with the term obesity reserved for the higher levels of weight excess. These levels of excess weight are defined on the basis of BMI, a measure of total body weight relative to height. Adults with a BMI of 18.5 to 24.9 are considered to have “normal” weight; the terms overweight, obese, and extreme obese are reserved for those with a BMI of 25 to 29.9, 30 to 39.9, and 40 and over, respectively. Children and adolescents ages 2 to 18 years with a BMI at the 95th percentile or above are considered obese, and those with a BMI between the 85th and 94th percentiles are considered overweight.45 Using metric units, BMI (kg/m2) is defined as weight in kilograms divided by height in meters squared (kg/m2). Using pounds and inches, BMI (kg/m2) is estimated as (Weight [lb]/Height [inches2]) × 703. Because BMI may overestimate the degree of excess body fat in some clinical situations (e.g., edematous states, extreme muscularity, muscle wasting, and short stature), the assessment of body composition in such cases often requires clinical judgment.
![]() Body mass index is an acceptable measure of obesity and is the practical method of defining obesity in the clinic and epidemiologic studies; however, it does not always correspond to excess fat. There are well-established differences in the relationship between BMI and obesity-related risks among disparate racial and ethnic groups. For examples, BMI overestimates adiposity among non-Hispanic blacks and underestimates risk among Asians.46–49 Ideally, obesity refers to a state of excess body fat as determined by measures of adiposity. Research techniques that can accurately measure fat mass separately from the body’s fat-free mass include determination of body density using underwater body weight or air displacement plethysmography, dual-energy x-ray absorptiometry, computed tomography (CT), and magnetic resonance imaging (MRI).50 These measurement techniques are currently too expensive and time-consuming to be used routinely in the clinical setting. Furthermore, all fat is not equal in its metabolic function or danger to health. Whereas brown adipose tissue promotes energy expenditure and has a weak association with metabolic markers of insulin production, release, and resistance, visceral white adipose tissue accumulation promotes energy storage and demonstrates a strong relationship with insulin resistance.42 Central obesity reflects high levels of intraabdominal or visceral fat, and this pattern of obesity is associated with an increased propensity for the development of hypertension, dyslipidemia, type 2 diabetes, and cardiovascular disease (sometimes referred to as the “metabolic syndrome”). Thus, in addition to the absolute excess fat mass, the distribution of this fat regionally in the body has important clinical effects. Intraabdominal fat is best estimated by imaging techniques such as CT and MRI but can be approximated through measurement of the waist circumference (WC). Clinically, WC is the narrowest circumference measured in the area between the last rib and the top of the iliac crest.51 The current definition for high-risk WC is greater than 40 inches (102 cm) in men and greater than 35 inches (89 cm) in women.51 Notably, epidemiologic studies demonstrate that WC adds little in terms of risk prediction after a patient’s BMI reaches 35 kg/m2. Thus, routine determination of WC should be implemented in those with BMIs between 25 and 34.9 kg/m2.
Body mass index is an acceptable measure of obesity and is the practical method of defining obesity in the clinic and epidemiologic studies; however, it does not always correspond to excess fat. There are well-established differences in the relationship between BMI and obesity-related risks among disparate racial and ethnic groups. For examples, BMI overestimates adiposity among non-Hispanic blacks and underestimates risk among Asians.46–49 Ideally, obesity refers to a state of excess body fat as determined by measures of adiposity. Research techniques that can accurately measure fat mass separately from the body’s fat-free mass include determination of body density using underwater body weight or air displacement plethysmography, dual-energy x-ray absorptiometry, computed tomography (CT), and magnetic resonance imaging (MRI).50 These measurement techniques are currently too expensive and time-consuming to be used routinely in the clinical setting. Furthermore, all fat is not equal in its metabolic function or danger to health. Whereas brown adipose tissue promotes energy expenditure and has a weak association with metabolic markers of insulin production, release, and resistance, visceral white adipose tissue accumulation promotes energy storage and demonstrates a strong relationship with insulin resistance.42 Central obesity reflects high levels of intraabdominal or visceral fat, and this pattern of obesity is associated with an increased propensity for the development of hypertension, dyslipidemia, type 2 diabetes, and cardiovascular disease (sometimes referred to as the “metabolic syndrome”). Thus, in addition to the absolute excess fat mass, the distribution of this fat regionally in the body has important clinical effects. Intraabdominal fat is best estimated by imaging techniques such as CT and MRI but can be approximated through measurement of the waist circumference (WC). Clinically, WC is the narrowest circumference measured in the area between the last rib and the top of the iliac crest.51 The current definition for high-risk WC is greater than 40 inches (102 cm) in men and greater than 35 inches (89 cm) in women.51 Notably, epidemiologic studies demonstrate that WC adds little in terms of risk prediction after a patient’s BMI reaches 35 kg/m2. Thus, routine determination of WC should be implemented in those with BMIs between 25 and 34.9 kg/m2.
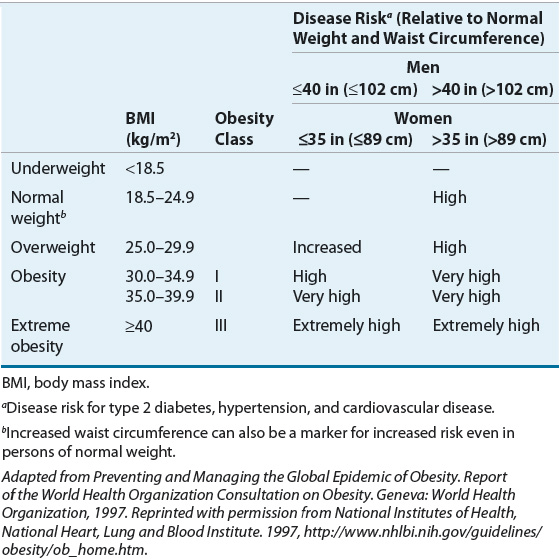
![]() Although BMI and WC are related, each measure independently predicts disease risk. Both measurements should be assessed and monitored during therapy for obesity.52 The risks for development of type 2 diabetes, hypertension, or cardiovascular disease at various stages of obesity based on BMI or WC are outlined in Table 121-3. Note that increased WC confers increased risk even in normal-weight individuals.
Although BMI and WC are related, each measure independently predicts disease risk. Both measurements should be assessed and monitored during therapy for obesity.52 The risks for development of type 2 diabetes, hypertension, or cardiovascular disease at various stages of obesity based on BMI or WC are outlined in Table 121-3. Note that increased WC confers increased risk even in normal-weight individuals.
TABLE 121-3 Classification of Overweight and Obesity by Body Mass Index, Waist Circumference, and Associated Disease Risk
Comorbidities
Although overall mortality rate is not increased among those classified as overweight, those who are obese have serious health risks and increased mortality rates,52 particularly adults with BMIs greater than 35 kg/m2.53 Substantial reductions in life expectancy have been predicted in adults with BMIs greater than 35 kg/m2.53 Further reduction in life span has been observed in obese individuals who are current or former smokers.53 Several disease states and conditions are more prevalent in obese patients (see Table 121-1).5–17 Increased body fat, increased total body weight, and a central distribution of body fat all are associated with an increased incidence of mortality, primarily as a result of cardiovascular disease. Hypertension, hyperlipidemia, insulin resistance, and glucose intolerance are all known cardiac risk factors that tend to cluster in obese individuals. Therefore, obese individuals are exposed to multiple risk factors. Some of the earliest studies from Framingham have confirmed the relationship between obesity and increased risk of stroke and coronary heart disease in both men and women.54 Blood pressure frequently is elevated in obese individuals and may in part explain the increased incidence of stroke and cardiovascular disease observed with obesity. Hypertension in lean individuals is associated with concentric cardiac hypertrophy as a consequence of an increased afterload, which increases the risk of cardiac ischemia. In contrast, eccentric dilation is observed in obesity, leading to an increased volume load. This dilated cardiomyopathy is associated with a reduction in ventricular ejection fraction and a high-output cardiac state. The combination of obesity and hypertension is associated with thickening of the ventricular wall, ischemia, and increased heart volume. This leads more rapidly to heart failure, an association that has been recognized for more than 2 decades.55,56 Alterations in pulmonary function are common in patients with obesity. Sleep apnea, which is more common in men, is a significant and costly condition that is associated with increased morbidity and mortality in obese individuals.55,56 The exact mechanism by which obesity leads to sleep apnea is unknown, but weight loss often results in significant and sometimes dramatic improvements in the condition.
Impaired glucose tolerance and Type 2 diabetes are associated with insulin resistance and obesity. As insulin response becomes impaired, the pancreatic β cells respond by increasing insulin production and release, resulting in a state of relative hyperinsulinemia. Although hyperinsulinemia is known to be associated with an increased risk of cardiovascular disease, it is not known whether the increased insulin levels contribute directly to cardiac disease or if they are a marker for the underlying defect of insulin resistance and glucose intolerance. Insulin resistance, in turn, also frequently leads to impaired lipid metabolism (increased cholesterol, increased triglycerides, and low circulating high-density lipoprotein) and hypertension. As with cardiovascular disease, central obesity is an important factor in determining the risk of developing type 2 diabetes.
Osteoarthritis in weight-bearing joints, such as the knees, may be related directly to the mechanical effects of excess body weight and the resulting forces exerted on these joint surfaces. The increase of osteoarthritis in non–weight-bearing joints, however, suggests that obesity may lead to altered cartilage, collagen, and even bone metabolism.8 Increasing evidence has suggested proinflammatory adipocytokines, such as tumor necrosis factor-α and leptin, may play an important role in the metabolic influence of overweight on osteoarthritis.57 Osteoarthritis and its symptoms, such as pain, are significant barriers to physical activity and key impediments to sustained weight loss.
Obesity affects the human reproductive system in a number of ways. Obesity is associated with earlier menarche in girls and hyperandrogenism, hirsutism, and anovulatory menstrual cycles in women. In some women, this disorder manifests as overt polycystic ovary syndrome, a condition in which insulin resistance is common.58 Weight loss therapy with an insulin sensitizer such as metformin has been shown to restore normal ovulation in some women.58 These observations suggest that insulin resistance plays a part in the causation of polycystic ovary syndrome associated with obesity.
TREATMENT
Available treatment options for the chronic management of obesity include reduced caloric intake, increased physical activity, behavioral modification, pharmacotherapy, and bariatric surgery.
Desired Outcomes
Weight management is commonly considered successful when a predefined amount of weight has been lost such that a final goal is achieved. However, desired outcomes are fully dependent on the clinical situation. The ultimate goals of treatment must be defined clearly. These goals may be absolute weight loss if obesity is present without other comorbid conditions. If improvement in blood glucose, blood cholesterol, and hypertension are primary goals, then these must be defined appropriately and may include setting target levels for low-density lipoprotein cholesterol, glycosylated hemoglobin, or blood pressure. Per current national guidelines, the recommended weight loss goal for adults is 10% of initial weight gradually over 6 months of therapy to achieve a reasonable rate of weight loss of about 1 to 2 lb (0.5–0.9 kg) per week.6 Success may also include end points of decreasing the rate of weight gain or maintaining a weight-neutral status. All too often patients expect to lose weight overnight, only to be disappointed. Thus, it is important to set a time course for the plan. A significant number of web-based resources for supporting both patient and practitioner weight management activities are available.22,23,51
General Approach to Treatment
The success of weight loss intervention has been measured most often as weight loss over a defined study period. Successful obesity treatment plans have incorporated an integrated dietary intervention, exercise, behavior modification (with or without pharmacologic therapy), and/or surgical intervention. Specific weight goals should be established that are consistent with medical needs and the patient’s personal desire. For most obese patients, a weight loss goal of 5% to 10% of initial weight is reasonable. Patients should not be allowed to attain an abnormally low body weight (i.e., less than their estimated ideal body weight).
Patients seeking help for obesity do so for many reasons, including improvement in their quality of life, a reduction in associated morbidity, and increased life expectancy. Unfortunately, numerous individuals seek therapy for obesity primarily for cosmetic purposes and often have unrealistic goals and expectations. Aggressive marketing of weight loss programs, therapies, and diets—parallel to the fashion industry’s standards of desirable body profiles—has led many individuals to set impossible goals and expectations. In some cases, these individuals will go to extreme measures to achieve weight loss. Consequently, clinicians must be careful to fully discuss the risks of therapies and to clearly define the achievable benefits and magnitude of weight loss. Obese patients should be redirected away from trying to achieve an “ideal weight” to the more reasonable goals of modest (e.g., loss of 5%–10% of body weight) but sustained, medically relevant weight loss. In practice, the goal has to be set based on many factors, including initial body weight, patient motivation and desire, presence of comorbid conditions, and age. For example, in patients with diabetes, even modest weight loss can improve glucose control and may reduce mortality rates,59–62 yet in individuals with osteoarthritis, significantly more weight reduction may be required to improve symptoms. Indeed, dietary modification and exercise have been shown to ameliorate hyperglycemia, hyperlipidemia, and hypertension with weight loss of less than 5% of initial body weight. The Look AHEAD (Action for Health in Diabetes) study found that patients with diabetes who maintained weight loss with lifestyle modifications for a period of 11 years did not experience a reduced incidence of cardiovascular events, but they did have a reduced need for diabetes medications and other positive health benefits.63 These data emphasize the importance of defining end points and measures of success in any weight loss plan.
![]() Weight loss interventions must be founded on lifestyle changes, such as a modification in eating practices; complemented by drug therapy, if indicated; and in some cases, surgery (Fig. 121-3). Before recommending any therapy, the clinician must evaluate the patient for the presence of secondary causes of obesity. If a secondary cause is suspected, then a more complete diagnostic workup and the initiation of appropriate therapy may be warranted. The next step in patient evaluation is to determine the presence and severity of other medical conditions that are either directly associated with obesity (e.g., diabetes) or that have an impact on therapeutic decision making (e.g., history of liver disease or cardiac arrhythmia). Appropriate laboratory tests to exclude or quantify the degree of specific conditions such as diabetes, liver dysfunction, and nephropathy should be performed as indicated by the history and physical examination. Based on the outcome of this medical evaluation, the patient should be counseled on treatment options, benefits, and risks. No matter what the treatment options are, they all require significant effort on the part of the patient to change lifestyle and comply with the management plan. If the patient is not yet ready to meet these expectations, then early counseling will reduce the chance of frustration for the patient; clinician; and in some cases, other family members. Providing basic education can lead to a significant change in motivation and desire to lose weight and improved compliance. Ultimately, lifelong therapeutic goals should consist of maintenance of reduced body weight and prevention of weight gain.
Weight loss interventions must be founded on lifestyle changes, such as a modification in eating practices; complemented by drug therapy, if indicated; and in some cases, surgery (Fig. 121-3). Before recommending any therapy, the clinician must evaluate the patient for the presence of secondary causes of obesity. If a secondary cause is suspected, then a more complete diagnostic workup and the initiation of appropriate therapy may be warranted. The next step in patient evaluation is to determine the presence and severity of other medical conditions that are either directly associated with obesity (e.g., diabetes) or that have an impact on therapeutic decision making (e.g., history of liver disease or cardiac arrhythmia). Appropriate laboratory tests to exclude or quantify the degree of specific conditions such as diabetes, liver dysfunction, and nephropathy should be performed as indicated by the history and physical examination. Based on the outcome of this medical evaluation, the patient should be counseled on treatment options, benefits, and risks. No matter what the treatment options are, they all require significant effort on the part of the patient to change lifestyle and comply with the management plan. If the patient is not yet ready to meet these expectations, then early counseling will reduce the chance of frustration for the patient; clinician; and in some cases, other family members. Providing basic education can lead to a significant change in motivation and desire to lose weight and improved compliance. Ultimately, lifelong therapeutic goals should consist of maintenance of reduced body weight and prevention of weight gain.
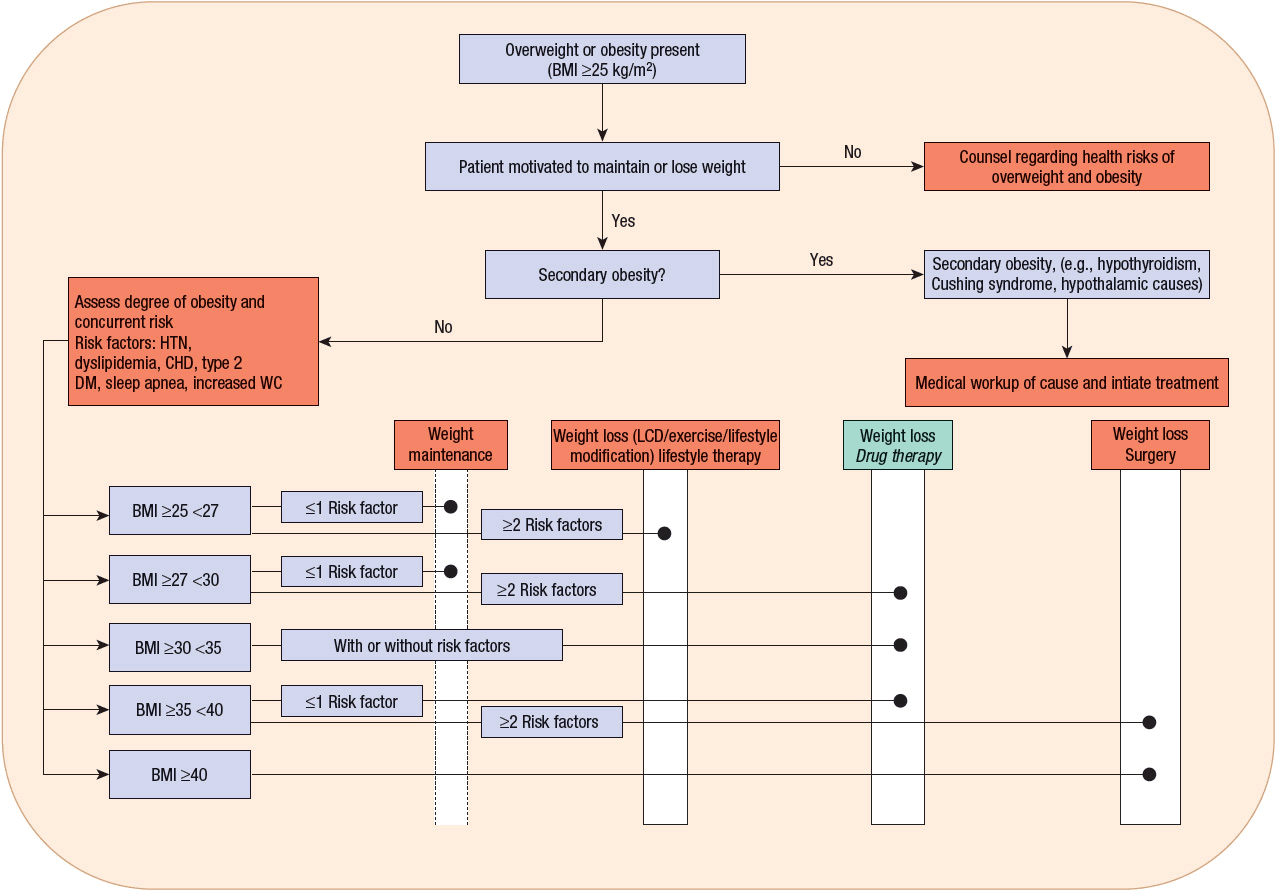
FIGURE 121-3 Treatment algorithm. Candidates for pharmacotherapy are selected on the basis of body mass index and waist circumference criteria along with consideration of concurrent risk factors. Medication therapy is always used as an adjunct to a comprehensive weight-loss program that includes diet, exercise, and behavioral modification. CHD, coronary heart disease; DM, diabetes mellitus; HTN, hypertension; LCD, low-calorie diet; WC, waist circumference (≥40 inches [≥102 cm] for men and ≥35 inches [≥89 cm] for women).
Nonpharmacologic Therapy
Nonpharmacologic therapy, including reduced caloric intake, increased physical activity, and behavioral modification, is the mainstay of obesity management. This combination is recommended as first-line therapy in current evidence-based clinical guidelines for the treatment of overweight and obesity in adults set forth by the NIH.6
Reduced Caloric Intake
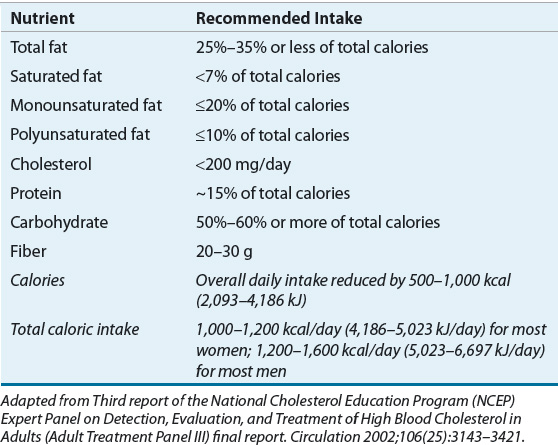
Current adult guidelines recommend reduced caloric intake through adherence to a low-calorie diet (LCD).6 The LCD should provide a daily caloric deficit of 500 to 1,000 kcal (2,093–4,486 kJ), which generally correlates to a total intake of 800 to 1,200 kcal/day (3,349–5,024 kJ/day). Severely obese individuals will require more energy, at least at the start of dietary restriction. The composition of the LCD is outlined in the Step I Diet recommended by the third report of the Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III), as shown in Table 121-4.64 Adherence to the LCD has been shown to result in an average weight loss of 8% after 6 months.6
TABLE 121-4 Recommended Composition of the Step I Low-Calorie Diet
Clinical Controversy…
Numerous diet and nutrition plans are available to aid patients in their pursuit of weight loss. Popular diets include moderate energy-deficient plans (e.g., Weight Watchers, LEARN [Lifestyle, Exercise, Attitude, Relationships, Nutrition], and Jenny Craig), vegetarian-based plans (e.g., Ornish), and low carbohydrate plans (e.g., Zone and Atkins). Short-term weight loss is significant for almost all diet plans. However, long-term weight loss and maintenance of weight loss are less promising, primarily because of difficulty with adherence. Low-carbohydrate diets have been found to achieve better weight loss than low-fat diets for the first 6 months of treatment, but similar efficacy is generally seen after 1 year.65,66 A long-term trial of more than 800 obese patients reported no significant difference in the amount of weight loss achieved with adherence to various types of reduced-calorie diets.67 Therefore, the macronutrient composition of the diet may not be as important as consistent adherence to reduced energy consumption.
Very-low-calorie diets, providing less than 800 kcal/day (3,349 kJ/day), are generally not recommended.6 Although very-low-calorie diets can often result in early weight loss, long-term results have been disappointing because it is difficult for individuals to maintain compliance.68 Additionally, very-low-calorie diets require intensive medical monitoring and should only be used in certain situations under the supervision of an experienced clinician. Regardless of the diet program, it is clear that energy consumption must be less than energy expenditure to achieve weight loss (see Fig. 121-1). The challenge is to develop a diet plan that leads to consistent adherence by the patient and sustained weight loss and maintenance.
Increased Physical Activity
Increased physical activity is an important component in achieving the state of greater energy expenditure than energy intake that is necessary to lose weight and maintain weight loss. When increased physical activity is attempted as monotherapy, only modest weight loss has been reported.69 However, when it is combined with reduced calorie intake and behavior modification, it can augment weight loss and improve obesity-related comorbidities and cardiovascular risk factors.6,69 Current adult recommendations suggest at least 150 minutes of moderate physical activity per week.70 However, 1 hour of moderate physical activity per day may be required to augment weight loss. Patients should be advised to start slowly and gradually increase intensity. All obese patients should receive a medical examination before embarking on a physical activity program.
Behavioral Modification
Behavior modification is common to almost all weight loss interventions. The primary aim is to help patients choose lifestyles that are conducive to safe and sustained weight loss. Behavioral therapy is based on principles of human learning and thus attempts to substitute desirable behaviors for learned undesirable habits using a combination of stimulus control and reinforcement. Most such programs use self-monitoring of diet and exercise both to increase patient awareness of behavior and as a tool for the clinician to determine patient compliance as well as patient motivation.24 Behavior is reinforced by techniques that include behavioral contracting, social support, self-monitoring, relapse prevention, and (in some cases) booster treatments. Behavioral contracts are written agreements jointly developed by patients and their clinicians. Components of these agreements include goals of therapy, methods to achieve these goals, and rewards for achieving these goals. Social support requires the active participation of a close friend or relative who is involved in monitoring compliance and reinforcing behavior. Relapse prevention is geared to identifying high-risk situations for relapse, such as social events, and developing strategies to overcome barriers to maintaining healthy lifestyle choices. The most effective behavioral interventions for weight loss include a combination of several components and involve multiple treatment sessions. Updated recommendations from the U.S. Preventative Services Task Force recognize that an intensive, multicomponent behavioral intervention program can result in improved glucose tolerance and reduced cardiovascular risk in obese patients.24
Bariatric Surgery
![]() Consistent with the growing obesity epidemic, the demand and popularity of bariatric surgery have increased drastically over the past 2 decades. Between 1990 and 2000, the annual prevalence of bariatric procedures grew nearly sixfold, from 2.4 to 14.1 per 100,000 adults.71 Each year, there are approximately 344,000 bariatric procedures performed worldwide.72 From 2004 to 2007, an increase of 148% in laparoscopic bariatric surgical procedures has been observed, and this number continues to rise.73
Consistent with the growing obesity epidemic, the demand and popularity of bariatric surgery have increased drastically over the past 2 decades. Between 1990 and 2000, the annual prevalence of bariatric procedures grew nearly sixfold, from 2.4 to 14.1 per 100,000 adults.71 Each year, there are approximately 344,000 bariatric procedures performed worldwide.72 From 2004 to 2007, an increase of 148% in laparoscopic bariatric surgical procedures has been observed, and this number continues to rise.73