Introduction
In worldwide terms, nutritional disorders are responsible for much morbidity and mortality. The three main categories of nutritional disorder are:
- undernutrition, which is dominated by insufficient food energy, producing the features of starvation
- malnutrition, which is deficiency of one or more of the essential nutrients
- obesity, which is excessive positive energy balance.
Disease is also possible as a result of nutrient excess (e.g. iron overload, alcohol excess) or the effect of potentially toxic agents in food (e.g. favism, an acute haemolytic anaemia due to sensitivity to the fava bean).
Nutritional issues, directly or indirectly, impinge upon many of the tests undertaken in clinical biochemistry. Many analytes are altered by nutritional status. For example, diet exerts important short-term effects on serum [triglyceride] and plasma [glucose], and longer term effects on serum [cholesterol]. Certain inborn errors of metabolism may demand special diets, which are monitored biochemically (e.g. phenylalanine in PKU). Less obviously, diagnostic tests may only be valid if certain nutritional requirements are met. For example, measurement of 5-HIAA requires exclusion of rich sources of serotonin from the diet (p. 219), screening for hypercalciuria requires an adequate calcium intake, etc. Laboratory measurements are also necessary in the management of patients receiving nutritional support, especially total parenteral nutrition (TPN), and in the assessment of malabsorption (e.g. faecal elastase). Suspected nutritional deficiencies, ranging from possible iron deficiency to vitamin or trace metal deficiencies, also require specialist laboratory tests.
In this chapter, the first section considers the principal nutritional constituents, including the clinical significance and measurement of vitamins and trace elements. This is followed by a section on nutritional support and finally a section on the contrasting problems of protein-energy malnutrition (PEM) and obesity.
Principal dietary constituents
The nutrients in food can be subdivided into the following categories:
- Energy (in the form of carbohydrate and fat)
- Protein (as a source of nitrogen and essential amino acids)
- Major minerals (notably potassium, sodium, magnesium, calcium)
- Micronutrients (13 essential vitamins and a number of trace elements).
Food also contains non-nutrients. These include, for example:
- Fibre
- Substances present as additives or arising from food production (e.g. preservatives, colouring, fungicides used to preserve fruit, growth hormones used in animal husbandry)
- Other non-nutritive substances which may add flavour, smell, colour, etc. to food.
Carbohydrates
The major source of dietary energy is normally provided by carbohydrate, in the form of sugars (e.g. sucrose, lactose) or digestible polysaccharides; the major food polysaccharide is starch, found in cereals, root vegetables and legumes. Non-digestible polysaccharides contribute to dietary fibre. Carbohydrates are not essential nutrients, but insufficient carbohydrate intake leads to ketosis. The energy supplied is 4 kcal/g. Dietary carbohydrate in excess of the body’s energy needs can be converted by the liver to fat. Stored carbohydrate is limited to muscle and liver glycogen, with the latter’s store depleted 18–24h after starvation. Complex carbohydrates contribute to the indigestible fibre content of the diet.
Fats
Dietary fat consists largely of triglycerides, with small amounts of other constituents (e.g. cholesterol). The National Food Survey (2000) showed that in Britain, on average, 35% of total energy was taken as fat and 48% was carbohydrate. Since the energy content of fat is greater than that of carbohydrate (~9 kcal/g, compared with 4 kcal/g for protein and for carbohydrate), the weight of dietary fat is substantially less than for carbohydrate on this diet.
Triglycerides contain saturated or unsaturated fatty acids, or both. Saturated fats are typically animal in origin, with epidemiological evidence that they predispose to cardiovascular disease possibly by raising LDL-cholesterol. Unsaturated fats are principally of plant or fish origin and tend to lower LDL-cholesterol and be cardioprotective, particularly in the case of the ω-3 unsaturated fatty acids found in fish oils (e.g. eicosapentenoic acid).
In terms of daily intake, the current recommendation for fat intake in the UK is that energy intake from fat should ideally be no more than 30% of total calorie intake with no more than a third provided as saturated fat.
Linoleic and linolenic acids comprise the ‘essential’ free fatty acids required for membrane synthesis and serving as precursosrs for prostanoid biosynthesis Arachidonic acid is also essential but can be made in vivo from linoleic acid.
Proteins
Dietary protein from both animal and plant sources is required as a source of nitrogen and the essential amino acids (eight of the 20 amino acids used in protein synthesis are essential). The carbon skeleton of the amino acids can also be a source of energy, and protein normally provides about 1114% of total calories on a 70–100 g protein diet; the minimum requirement is 40 g of protein of good biological value. Vegetable protein may be deficient in one or more of the eight essential amino acids, but this deficiency can be overcome by complementation, whereby a combination of cereals and legumes together provide protein of good biological value. As with carbohydrate, the energy supplied is 4 kcal/g.
Trace elements
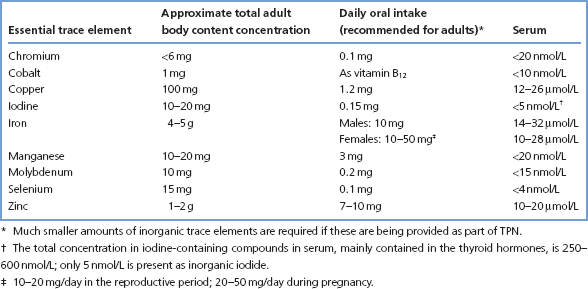
More than 20 elements are known to be essential in animal nutrition. Of these, seven are ‘bulk’ elements (Na, K, Ca, Mg, Cl, S and P) and the rest are referred to as trace elements, present in tissues at less than 100 ppm. Table 15.1 lists some data about those that are known as essential trace elements.
The clinical importance of iron (e.g. in haem), iodine (in thyroid hormones) and cobalt (in cobalamin) is well established. Clinical syndromes associated with deficiency of copper, selenium, zinc, chromium, manganese and molybdenum have all been described, and these six elements will be considered here. The effects of iodine and iron deficiency are described elsewhere (pp. 118 and 258). Deficiency of inorganic cobalt has not been reported in man.
Table 15.1 Trace elements essential to the human body
Deficiencies of essential trace elements usually arise in association with PEM, or with other abnormal nutritional states (e.g. TPN, neonatal feeds, synthetic diets). Specific inherited disorders in trace element handling are rare. Excessive losses, especially in association with severe and chronic GI diseases, may also cause deficiency.
Methods of assessing essential trace element deficiency are not straightforward and depend upon specialised techniques available in a limited number of laboratories. Moreover, plasma concentrations may not necessarily reflect actual nutritional status for a particular trace element, where intracellular levels may be more relevant. Despite these limitations, plasma values are often taken to indicate a specific deficiency. Changes in concentration may also reflect changes in concentration of plasma proteins that bind the metals. Diagnosis is often only made retrospectively on the basis of a clinical condition likely to have given rise to deficiency, occurring in association with clinical symptoms that can be attributed to the lack of trace elements, and which responds to treatment with the appropriate supplementation.
Zinc, copper and selenium
These micronutrients are more commonly monitored than other trace elements in the assessment of nutritional status, largely because of established deficiency syndromes associated with them.
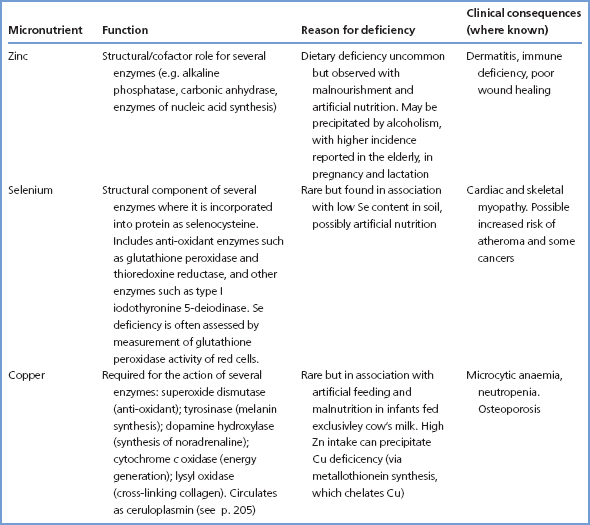
Table 15.2 summarises some of the known function of these micronutrients and the causes and consequences of deficiency.
Absorption of zinc from the intestine appears to be controlled in a manner similar to that of iron (p. 255), with sequestration of zinc in enterocytes, as metallothionein, and transfer of some of this to the plasma; the rest is lost when the enterocytes are sloughed. Zinc is mostly transported bound to albumin, α2-macroglobulin and transferrin. Specimens of blood for zinc measurement are affected by feeding and venous stasis; serum [zinc] may fall by as much as 20% after meals and levels should be assessed under fasting conditions. The body does not store zinc to any appreciable extent in any organ; urinary excretion is fairly constant at 10 μmol/24 h, with re-excretion into the gut being the main route for adjusting the amount excreted. Zinc excretion can, however, increase in trauma and catabolic states as an accompaniment to muscle protein catabolism and increase the risk of zinc deficiency. Injury, surgery, infection and a variety of acute illnesses are often accompanied by a fall in serum [zinc] due to the stimulation of hepatic metallothionein synthesis; this is one of the many components of the acute-phase response (p. 240). Measurement of CRP may point to an acute-phase response as the cause of a low serum zinc. Zinc levels may also fall in malignant disease or chronic liver disease without clinical zinc deficiency.
Table 15.2 Known functions of zinc, copper and selenium, and causes and consequences of deficiency
Other trace elements
Chromium may be involved in glucose homeostasis; a chromium complex present in brewer’s yeast (‘glucose tolerance factor’) is able to improve glucose tolerance in some diabetics. Malnourished infants may develop severe glucose intolerance that improves with chromium supplementation. In adults, a syndrome presenting with weight loss, peripheral neuropathy and marked insulin-insensitive glucose intolerance has been described that improves with chromium supplementation.
Manganese is a component of certain metalloenzymes, and manganese ions activate a large number of other enzymes, for example those involved in the synthesis of glycosaminoglycans, cholesterol and prothrombin. Despite this extensive range of enzyme requirements for manganese, true deficiency in humans appears to be very rare.
Molybdenum is a component of xanthine oxidase and some other metallo-enzymes. Its deficiency has been reported to cause xanthinuria, with low serum [urate] and low urinary uric acid output.
Vitamins
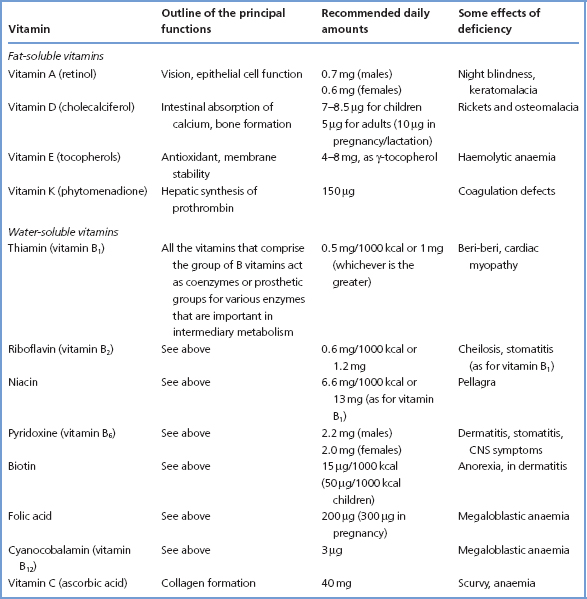
Vitamins are all organic compounds that, as originally defined, cannot be synthesised in the human body and must be provided in the diet. They are essential for the normal processes of metabolism, including growth and maintenance of health. It is now known that the body is able to produce part or even all of its requirements for some of the vitamins, for example vitamin D from cholesterol and niacin from tryptophan. Table 15.3 summarises some data concerning both water-soluble and fat-soluble vitamins.
Table 15.3 The vitamins
Deficiency may arise from inadequate diet, impaired absorption, insufficient utilisation, increased requirement or increased rate of excretion. Vitamin deficiency develops in stages:
Biochemical investigations help to confirm the diagnosis of some overt vitamin deficiency diseases, and may allow the diagnosis to be made at an earlier stage. Several types of biochemical tests are available, of which only some will be applicable to the investigation of suspected deficiency of a particular vitamin. The vitamin may be directly measured in whole blood, plasma or serum, erythrocytes, leucocytes or tissue biopsy specimens. Alternatively, direct measurement of the vitamin or one of its major metabolites in urine is possible. In general, plasma concentrations of vitamins do not necessarily reflect the vitamin status of the body. Measurements of vitamins in cells generally give a much better indication of the body’s vitamin status. Plasma levels usually fall before cellular and tissue levels fall, but low or undetectable plasma levels can occur in the absence of deficiency. Conversely, recent dietary intake can cause the plasma concentrations of vitamins to fluctuate markedly, even in severe deficiency. However, a sustained high plasma [vitamin] usually excludes a deficiency state.
Alternative approaches to assessment of vitamin status focus on the cofactor function of the vitamin. These include cofactor saturation tests in which the activity of the enzyme is measured in vitro before and after the addition of the enzyme’s vitamin cofactor or prosthetic group. Clinical suspicion of a vitamin deficiency can also be corroborated by observing a response to the relevant vitamin supplementation; however, in the absence of measurements, the level of certainty is lower.
Deficiency of fat-soluble vitamins
Vitamin A
This vitamin is present in the diet as retinol. It can also be derived from dietary β-carotene, some of which is hydrolysed in the intestine to form retinol. A rich source is liver, although leafy vegetables and some fruits also provide the vitamin in large amounts. After absorption, followed by esterification in the mucosal cells, the ester is transported in the blood by retinol-binding protein. Specific binding proteins on cell membranes are involved in the uptake of vitamin A ester from plasma into the tissues. The vitamin is stored in the liver, mainly as its ester.
The active form of vitamin A, 11-cis-retinal, is necessary for rod vision, and its deficiency can cause night blindness. Another form, retinoic acid, induces differentiation of epithelial cells. Vitamin A deficiency predisposes to GI and respiratory tract infections and leads to night blindness and, if severe, to keratinisation of the cornea, corneal ulceration and, ultimately, blindness. Serum [vitamin A] may be decreased in states of severe protein deficiency, due to lack of its carrier protein, and may then increase if the protein deficiency is corrected.
Laboratory measurement is carried out by determination of serum [vitamin A], but this provides only limited information about the state of the tissue stores.
Vitamin D
The formation and metabolism of vitamin D are described on p. 73. Rickets in infancy and child-hood, and osteomalacia in adults are the main forms of vitamin D deficiency (p. 83).
Vitamin E
Eight related tocopherols and tocotrienols possess vitamin E activity; they have antioxidant properties, and protect against oxidant (free radical) damage to polyunsaturated fatty acids in cell membranes.
Vitamin E deficiency is a rare complication of prolonged and severe steatorrhoea, and of prolonged parenteral nutrition. Altered red cell membrane stability can lead to haemolytic anaemia in children, while skeletal muscle breakdown may be responsible for the raised serum CK activity observed in both adults and children. Neurological consequences have also been described. Deficiency is investigated by measuring plasma [vitamin E].
Vitamin K
Vitamin K is not only found in liver and leafy vegetables (as K1 or phylloquinone) but is also synthesised by colonic bacteria (as K2 or menaquinone). It is necessary for the post-translational modification in proteins of glutamate side chains by γ-carboxylation. The presence of a second carboxyl group on the glutamate side chain confers phospholipid-binding properties on the modified protein in the presence of Ca2+. Proteins containing γ-carboxyglutamate include certain clotting factors (II, VII, IX and X) and the bone matrix protein, osteocalcin.
Vitamin K deficiency is most often due to treatment with anticoagulants (e.g. warfarin); it leads to reduced levels of the vitamin K-dependent coagulation factors and, hence, to haemorrhage. Deficiency may also arise in obstructive jaundice, and levels may be low in the newborn (leading to haemorrhagic disease of the newborn). Tests to assess vitamin K status include the PT – an important test in the investigation and management of jaundiced patients (p. 199) and of those on anticoagulant treatment.
Deficiency of water-soluble vitamins
Ascorbic acid (vitamin C)
Vitamin C deficiency leads to scurvy which is characterised by perifollicular haemorrhages, swollen gums with loosened teeth, bruising, spontaneous haemorrhages and anaemia. Frank scurvy rarely occurs nowadays, but its subclinical form is by no means uncommon, especially among elderly people living alone. Ascorbic acid is a water-soluble anti-oxidant which maintains iron in the reduced (ferrous) form and which is essential to the activity of lysine and prolyl oxidase which cross-link collagen. Rich sources include citrus fruits, blackcurrants and potatoes.
Plasma [ascorbate] measurements provide a poor index of tissue stores. Leucocyte ascorbate measurements provide a reasonable assessment of tissue stores of ascorbate, but difficulties in obtaining leucocytes uncontaminated by other cellular elements mean that the buffy layer, consisting of leucocytes and platelets (and a few erythrocytes), is normally examined instead. Leucocytes and platelets take up ascorbate against a concentration gradient, and may retain most of their ascorbate even when plasma [ascorbate] has fallen to undetectable levels. Buffy layer [ascorbate] falls at about the same time as clinical evidence of scurvy appears, and seems to give a good indication of the body’s stores of the vitamin. In clinical practice, suspicion of vitamin C deficiency may prompt a trial of vitamin C supplementation without formal measurement of vitamin C status.
Thiamin (vitamin B1)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


