Chapter 10 Nucleotide Synthesis and Metabolism
I. Overview
A. Nucleotides are composed of ribose phosphate (donated by phosphoribosyl pyrophosphate [PRPP]) and a purine or pyrimidine base.
B. Several kinases use adenosine triphosphate (ATP) to generate nucleoside diphosphates and triphosphates.
D. Pyrimidines are synthesized by condensation of carbamoyl phosphate and aspartate; the ribose phosphate is donated later in the process.
E. Synthesis of purines and pyrimidines is allosterically regulated to produce balanced amounts of each nucleotide.
F. Degradation pathways are designed to salvage some nucleotides and to produce uric acid (purines).
II. Nucleotide Structure
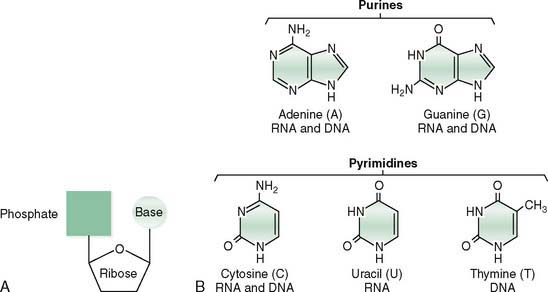
A. Nucleotides consist of an organic base bound by an N-glycosyl linkage to a phosphorylated pentose (Fig. 10-1A).
B. The nucleotide base has a purine (adenine, guanine) or pyrimidine (cytosine, thymine, uracil) ring structure (see Fig. 10-1B).
C. The nucleotide pentose is ribose in RNA or deoxyribose in DNA.
1. Ribonucleotide reductase reduces nucleoside diphosphates (ADP, GDP, CDP, and UDP) to their deoxy forms (e.g., dADP) in a reaction that requires reduced thioredoxin as a cofactor.
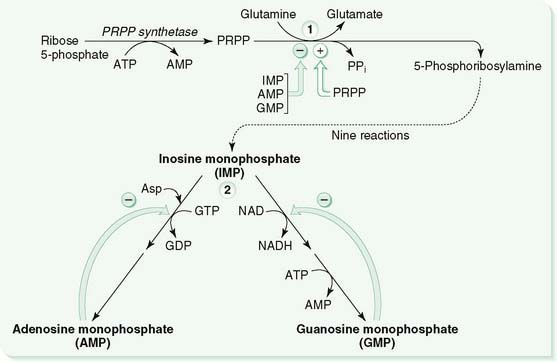
III. Purine Synthesis (Fig. 10-2)
A. Step 1
1. Formation of 5-phosphoribosylamine from 5-phosphoribosyl 1-pyrophosphate (PRPP) and glutamine is a committed step in the de novo synthesis of purines (see Fig. 10-2).
2. End-product feedback inhibition of this reaction by guanosine monophosphate (GMP), adenosine monophosphate (AMP), and inosine monophosphate (IMP) prevents overproduction of purines; high PRPP concentrations overcome this inhibition.
B. Step 2
1. Conversion of IMP to AMP or GMP is controlled by two mechanisms to achieve balanced production of AMP and GMP.
2. One mechanism is cross-regulation, in which the end product of one pathway is required for the other pathway.