Fred P. Gattas
6-1. Key Points
■ Radiopharmaceuticals are mainly used for diagnostic purposes, but can also be used for palliative and therapeutic indications.
■ Technetium Tc-99m is eluted from a molybdenum Mo-99/Tc-99m generator at the pharmacy and can be used as a compounded ingredient or directly injected as a radiopharmaceutical.
■ Cardiac imaging is performed to help visualize perfusion, calculate the ejection fraction and ventricular wall motion, and help diagnose myocardial infarction.
■ Cerebral imaging is performed to help detect perfusion in stroke patients, diagnose brain death, and help identify Parkinsonian syndrome. It can also be used to visualize cerebral spinal flow patterns and help diagnose cognitive impairment by estimating the density of beta-amyloid neuritic plaque.
■ Radiolabeled white blood cells or gallium citrate Ga-67 may be intravenously injected to help detect sites of infection.
■ Radiopharmaceuticals are used to help identify tumors that are metabolically active or have a high density of somatostatin receptors. They can identify prostate cancer cells and image pheochromocytomas or neuroblastomas.
■ Radiopharmaceuticals can also help remove residual tumor tissue in the brain and are also used therapeutically to treat non-Hodgkin lymphoma.
■ Pulmonary imaging is performed for two purposes: ventilation and perfusion.
■ Tc-99m albumin aggregated (Tc-99m MAA) is the radiopharmaceutical for pulmonary perfusion studies.
■ Xenon (Xe)-133 gas and Tc-99m pentetate (Tc-99m DTPA) aerosol can be used for ventilation studies.
■ Renal scintigraphy can be divided into the following categories: perfusion imaging, function studies, evaluation for renal artery stenosis, and cortical imaging.
■ The three radiopharmaceuticals used in renal studies are Tc-99m DTPA, Tc-99m mertiatide (Tc-99m MAG3), and Tc-99m succimer (DMSA).
■ Nonradioactive interventional pharmaceuticals can be used to enhance the diagnostic accuracy of these studies.
■ Tc-99m sulfur colloid is indicated for reticuloendothelial system (RES) scintigraphy.
■ Tc-99m disofenin or Tc-99m mebrofenin are both indicated for hepatobiliary imaging, and nonradioactive interventional agents can be used with these radiopharmaceuticals.
■ Gastric emptying and motility studies can be performed with Tc-99m sulfur colloid incorporated into various foods.
■ Gastroesophageal reflux studies can be performed with Tc-99m sulfur colloid or In-111 pentetate (In-111 DTPA) compounded into liquids.
■ Diagnostic skeletal imaging studies can be performed with either a Tc-99m-labeled diphosphonate compound or F-18 sodium fluoride.
■ Palliative therapeutic radiopharmaceuticals for the treatment of bone pain from metastatic lesions include strontium-89 chloride and samarium Sm-153 lexidronam (Sm-153 EDTMP).
■ Tc-99m sodium pertechnetate, I-123 sodium iodide, or I-131 sodium iodide can be used for diagnostic studies of the thyroid gland.
■ I-123 sodium iodide and I-131 sodium iodide are used in whole body imaging for the detection of metastatic sites from thyroid cancer.
■ I-131 sodium iodide can be used as a therapeutic radiopharmaceutical for the treatment of hyper-thyroidism or the ablation of metastatic sites originating from thyroid carcinoma.
6-2. Study Guide Checklist
The following topics may guide your study of this subject area:
■ The difference between diagnostic and therapeutic medications
■ Mechanism of action for the medications
■ Knowledge of which radiopharmaceuticals are compounded and which are procured from manufacturers
■ Food, drug, and disease state interactions that can alter study results
■ When performing a pair of nuclear imaging studies, which one should be performed first
■ Specific dosing for pediatric patients
6-3. Introduction
Nuclear pharmacy is the practice of using radio-nuclides as a component of a diagnostic, palliative, or therapeutic drug. The difference largely involves the decay scheme of the radioactive nuclides. Radio-nuclides decay by different schemes, and each emission involves a signature energy measured in electron volts (eV). Radionuclides can decay and emit gamma and x-ray photons that can be used for imaging. Some radionuclides emit alpha or beta particles that have mass and cause cellular damage, and some have both gamma and alpha or gamma and beta emission. Some radionuclides emit positrons that are useful in imaging, such as in positron emission tomography (PET) (see Table 6-1).
All radionuclides have a physical half-life that follows first-order kinetics: A1 = A0e–λt, where A1 = activity after a certain time in the future, A0 = initial activity, λ = 0.693/half-life time, and t = time of decay. Note that both times must be expressed in the same units. Activities of radiation for administration in the United States are expressed in curie (Ci) units.
Table 6-1. Properties of Radionuclides
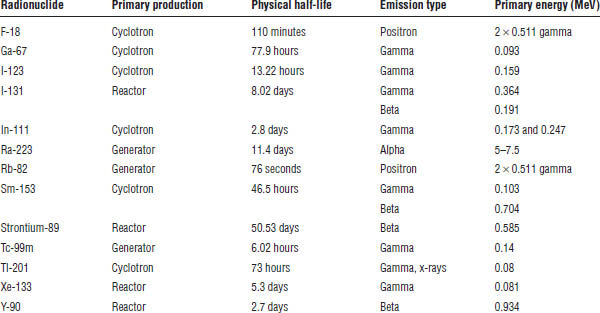
Some radionuclides and the ligands to which they are sometimes tagged are produced by a commercial manufacturer in a cyclotron (i.e., thallous chloride Tl-201 and indium In-111 oxine). Some radio-nuclides are produced by a commercial manufacturer in a nuclear reactor (i.e., I-131). Some radionuclides are produced by a commercially available generator on site (i.e., technetium Tc-99m and rubidium Rb-82).
Commercially available nonradioactive ligands (kits) approved by the U.S. Food and Drug Administration (FDA) are available for inventory storage from various manufacturers, and the compounder can label the ligands with a radionuclide. The radio-nuclide allows detection or the therapy of the localization site of the radioactive ligand complex. Most labeling uses Tc-99m, In-111, fluorine F-18, or yttrium Y-90 as the radionuclide, with Tc-99m being in the vast majority. Most ligand kits require stannous chloride to reduce the Tc-99m from the +7 valence state to a lower one for radiolabeling. Radio-chemical purity tests must be performed for all radio-labeled kits to ensure purity is within United States Pharmacopeia (USP) specifications.
F-18, In-111, and Y-90 are supplied from FDA-approved manufacturers. Tc-99m is supplied from eluting a molybdenum Mo-99/Tc-99m generator. The generator expires 2 weeks postcalibration and is available in a variety of activities.
Tc-99m Sodium Pertechnetate (Na99mTcO4)
Radiopharmaceutical Na99m TcO4 is produced from a Mo-99/Tc-99m generator. The generator has Mo-99 in various activities loaded onto an alumina column by the manufacturer. Tc-99m is produced via Mo-99 decay. When the generator is received, a 0.9% saline charge is placed on one port of the generator, and a shielded, sterile, evacuated vial is placed on the other port; the tubing is connected through the Mo-99 column. The evacuated vial pulls the normal saline through the column, the pertechnetate ion is displaced from the column by the chloride (Cl−) ion, and the evacuated vial fills with Na99mTcO4 solution that is isotonic in nature. That solution is used either to label a variety of different ligands or to be directly injected as a radio-pharmaceutical. Radiochemical purity, chemical purity, and radionuclidic purity tests should be performed to ensure the product is within USP specifications (see Table 6-2).
Table 6-2. Tc-99m Pertechnetate USP Purity Specifications
Purity | Specification |
Radiochemical purity | 95% |
Radionuclidic purity | 0.15 μCi of Mo-99/1 mCi of Tc-99m at administration |
Chemical purity | 10 mcg of Al/mL |
USP, United States Pharmacopeia.
6-4. Cardiac Imaging
Cardiac imaging can be performed with planar imaging, single photon emission computed tomography (SPECT), or PET. These cardiac studies provide information on perfusion, function, viability, and metabolic activity. A variety of radiopharmaceuticals are used, and these studies may be performed at rest or during stress. Stress studies can be performed through exercise, pharmacologic intervention, or both.
Pharmacologic stress agents include the following:
■ Dipyridamole
■ Adenosine (Adenoscan)
■ Regadenoson (Lexiscan)
■ Dobutamine
Thallous Chloride Tl-201
Thallous chloride Tl-201 is a monovalent cation and an analog of potassium that accumulates in viable myocardium through the Na+/K+ ATPase pump. It is useful in the diagnosis of coronary artery disease for assessing myocardial perfusion. By performing rest and stress studies, diagnosticians can distinguish reversible ischemia from irreversible ischemia or infarct. The intravenous (IV) radiopharmaceutical dosage ranges from 2 to 4 mCi. Tl-201 must be administered before a Tc-99m heart agent if performing dual isotope imaging.
Fludeoxyglucose F-18 (F-18 FDG)
F-18 is a positron emitter, and F-18 FDG is a metabolic indicator of glucose uptake in cells. Both glucose and F-18 FDG undergo active transport into cells by using glucose transporters, but F-18 FDG becomes trapped inside the cell once it is phosphorylated. Because this agent demonstrates metabolic activity, it is useful in determining viability of the myocardial tissue. It is used in combination with a myocardial perfusion radiopharmaceutical study to determine whether a patient will benefit from revascularization of coronary arteries. The IV dosage ranges from 5 to 15 mCi. Because F-18 is a cyclotron product with a 110-minute physical half-life, proximity to the site of production can be a limitation in acquiring this radiopharmaceutical.
Tc-99m Sestamibi (Tc-99m MIBI, Cardiolite)
Tc-99m sestamibi is a monovalent cationic complex, but unlike Tl-201, it is not a potassium analog. It is a lipophilic complex that undergoes passive diffusion into the myocardium and becomes attached to myocyte mitochondria. It is thought to be an indicator of both viability and perfusion. It is indicated for the identification of reversible myocardial ischemia and infarction and for the assessment of myocardial function. The recommended IV dosage range for this agent is 10 to 30 mCi, and a separate injection is required for both the rest and the stress studies.
Tc-99m Tetrofosmin (Myoview)
This radiotracer is a lipophilic, monovalent cationic complex that does not involve the Na+/K+ ATPase pump for localization into the myocardium. It provides information on both myocardial perfusion and left ventricular function. Tc-99m tetrofosmin scinti-graphic imaging is performed both at rest and under stress, and this procedure identifies regions of reversible myocardial ischemia whether or not myocardial infarct is present. The recommended IV dosage range for this tracer is 5 to 33 mCi, and a separate injection is required for both the rest and the stress studies.
Rb-82 Chloride
Rb-82 chloride is a PET radiopharmaceutical produced in a commercially available strontium (Sr)-82/Rb-82 generator. Sr-82 is the parent radionuclide, and it decays to Rb-82. The generator is eluted with normal saline, and the Rb-82 chloride is administered directly to the patient from the generator via an IV infusion system. Rb-82 is a potassium analog, and it is transported inside the myocyte by the Na+/K+ ATPase pump; thus, uptake is greater in viable myocardium, whereas there is an absence of radioactivity in nonviable tissue. This radiopharmaceutical in combination with PET is used for the assessment of regional myocardial perfusion in patients known to have or suspected of having coronary artery disease, and it can be used in rest or with pharmacologic stress studies. Two separate doses are required for rest and stress myocardial perfusion imaging, and 30–60 mCi is the range for a single dose of Rb-82 chloride.
UltraTag RBC (Tc-99m-Labeled Red Blood Cells)
Whole blood in 1–3 mL amounts is added to the reaction vial containing stannous ion. The stannous ion is distributed into the red blood cells via passive diffusion, and other components are added to inactivate extracellular tin. Then, 10–100 mCi of Tc-99m Na99mTcO4 is added. Once the Tc-99m enters the red blood cells, it is reduced by the tin and prevented from diffusing out of the cell. Labeling yields average about 95%. Then, 10–20 mCi of whole blood containing the radioactive red blood cells is reinjected back into the patient to visualize blood pool imaging, including cardiac first pass and gated equilibrium imaging. Evaluation of ventricular function is performed by having the patient attached to an electrocardiogram (ECG) synchronizer and timing the camera images to the R wave. Determination of left ventricle ejection function of the heart in addition to the wall motion during contraction can be accomplished with this study.
Tc-99m Pyrophosphate (Tc-99m PYP)
In addition to labeling the red blood cells in vitro, in vivo radiolabeling can be performed with Tc-99m PYP. A PYP vial is reconstituted with normal saline, and 15 mcg of stannous per kilogram of body weight is injected into the patient. After 15–30 minutes, the Tc-99m is injected and the red blood cell–labeling occurs. Labeling yields range from 60% to 80%.
A modified in vivo method can also be performed to increase labeling efficiency to approximately 90% by pulling 3–5 mL of the “tinned” blood into a syringe and adding Tc-99m Na99mTcO4 via a three-way stop cock.
Myocardial Infarct Imaging with Tc-99m PYP
To help diagnose a myocardial infarction, one can inject 24–48 hours postinfarct, 20 mCi of Tc-99m PYP into a patient. After cell death from a myocardial infarction, calcium is released from the cardiac mitochondria with the resulting calcium phosphate complexes allowing Tc-99m PYP binding. Note that at least minimal blood flow to the affected necrotic tissue is necessary to deliver the drug. Imaging can take place from 1 to 4 hours after administration.
6-5. Central Nervous System Imaging
Brain imaging or scintigraphy is performed to assess regional cerebral perfusion in suspected stroke patients, to assist in the clinical diagnosis of brain death, to determine an alteration in the blood–brain barrier (BBB), or to visualize striatal dopamine transporter in adult patients suspected of having Parkinsonian syndromes. Cisternography can be used to evaluate the flow pattern of the cerebrospinal fluid, and PET can be used to image beta-amyloid neuritic plaques in patients exhibiting cognitive impairment.
Cerebral Perfusion in Suspected Stroke Patients
Tc-99m exametazime (Ceretec)
One of the indications for Tc-99m exametazime is for cerebral scintigraphy as an adjunct in the identification of altered regional cerebral perfusion in stroke patients. The active moiety is the lipophilic Tc-99m complex, which is capable of crossing the BBB. The maximum brain uptake is 3.5–7%, and this occurs within 1 minute post-IV administration. The recommended average adult (70 kg) dosage is 10–20 mCi of Tc-99m exametazime. When methylene blue is used as a stabilizer, the preparation will be blue in color. A filter is attached to the syringe, and the preparation is filtered during the injection. When used to differentiate between ischemic and infarct areas, acetazolamide acts as an interventional agent to assess hemodynamic reserve.
Tc-99m bicisate (Neurolite)
This agent is indicated for use with SPECT as an adjunct to magnetic resonance imaging or conventional computed tomography (CT) in determining the site of stroke in patients previously diagnosed with a stroke. Tc-99m bicisate crosses an intact BBB via passive diffusion as a result of its lipophilic characteristic. In normal volunteers, cellular uptake in the brain at 5 minutes was reported to be 4.8–6.5%. The recommended IV radiopharmaceutical dosage for a patient weighing 70 kg is 10–30 mCi.
Clinical Diagnosis of Brain Death
Tc-99m pentetate (Tc-99m DTPA)
Because this chelate cannot cross an intact BBB, it is characterized as a nondiffusible radiopharmaceutical. An off-label use is the assessment of dynamic blood flow in the brain to assist in evaluation of a patient suspected of being brain dead. Brain death correlates with an absence of cerebral perfusion upon IV administration of 15–20 mCi of the radiopharmaceutical.
Tc-99m exametazime (Ceretec)
The use of this tracer to assist in determining brain death is an off-label use. In contrast to Tc-99m DTPA, interpretation of brain death with Tc-99m exametazime relies more on the evaluation of parenchymal brain uptake on delayed imaging; however, dynamic blood flow in the brain can also be identified with this radiopharmaceutical. The presence of radioactivity in the brain with time indicates brain perfusion. The IV dosage of this tracer is 10–30 mCi.
Tc-99m bicisate (Neurolite)
Just as Tc-99m exametazime is a diffusible brain-imaging agent, so is Tc-99m bicisate. An off-label use of this radiopharmaceutical is for assisting in the determination of brain death. Brain imaging with this tracer provides both dynamic blood and parenchymal uptake information. The conservation of brain perfusion is indicated by accumulation of radioactivity in the brain. The IV dosage of this radiopharmaceutical is 10–30 mCi.
Suspected Parkinsonian Syndromes
I-123 ioflupane (DaTscan)
Not only is this a radiopharmaceutical, but it is also a controlled drug (C-II). With SPECT imaging, I-123 ioflupane is used for striatal dopamine transporter visualization to aid in the assessment of adult patients with suspected Parkinsonian syndromes. It is helpful in distinguishing essential tremor from tremor resulting from Parkinsonian syndromes, and this tracer is considered an adjunct to other diagnostic modalities. A thyroid-blocking agent should be administered to the patient at a minimum of 1 hour prior to radio-pharmaceutical administration. The IV dosage of I-123 ioflupane is 3–5 mCi.
Cisternography
In-111 pentetate (In-111 DTPA)
In-111 DTPA is the only radiopharmaceutical with FDA approval for cisternography studies. After intrathecal administration, the radiopharmaceutical follows the path of the cerebrospinal fluid and can be used to identify abnormal flow patterns, including cerebrospinal fluid rhinorrhea and otorrhea. In a normal patient, approximately 65% of the administered radiopharmaceutical is excreted via the kidneys within 24 hours. In the average 70 kg patient, the maximum radiopharmaceutical dosage is 0.5 mCi.
PET
Florbetapir F-18 (Amyvid)
Florbetapir F-18 injection received FDA approval in April 2012 for PET brain imaging to estimate the density of beta-amyloid neuritic plaque in patients being evaluated for Alzheimer’s disease and other causes of cognitive impairment. PET with florbetapir F-18 is considered an adjunctive diagnostic procedure to other diagnostic modalities, and a positive study with this radiopharmaceutical does not establish a diagnosis of a cognitive disorder. The recommended dosage of this PET radiopharmaceutical is 10 mCi in 10 mL of volume or less.
6-6. Infection and Inflammatory Imaging
Infection and inflammatory-site imaging are performed with either gallium citrate (Ga-67) or auto-logous radiolabeled leukocytes. The clearance of the radiopharmaceuticals from nontarget areas determines when imaging or scintigraphy commences.
In-111 Oxyquinoline (In-111 oxine)
This diagnostic radiopharmaceutical is used to radiolabel autologous white blood cells as an adjunct in localizing sites of infection. After blood is withdrawn from a patient, the white blood cells are separated and then radiolabeled with In-111 oxine via passive diffusion. The radiolabeled leukocytes are then intravenously administered to the patient. The rationale for this procedure is that leukocytes are attracted to sites of infection and inflammation; thus, radio-labeled leukocytes in combination with scintigraphy can be useful adjuncts in identifying these sites. An adequate number of leukocytes is critical for study sensitivity; thus, patients who are leukopenic are not good candidates. The recommended dosage is 0.2–0.5 mCi of In-111 oxine–labeled autologous leukocytes. The labeled cells should be reinjected into the patient no later than 5 hours after the initial blood harvesting.
Tc-99m Exametazime (Tc-99m Ceretec)
In addition to cerebral imaging, Tc-99m exametazime is indicated for leukocyte-labeled scintigraphy as an adjunct in the localization of intra-abdominal infection and inflammatory bowel disease. White blood cell separation is the same as for In-111 oxine–labeled cells, and the normal dosage is 7–25 mCi. Optimal imaging is 2–4 hours postadministration.
Gallium Citrate (Ga-67)
Gallium citrate is beneficial in aiding in the detection of an infectious site in patients with fever of unknown origin, in assisting with the diagnosis of opportunistic infections, and in identifying some inflammatory sites. Because one cannot distinguish between tumor uptake and inflammatory sites, additional diagnostic procedures must be used to delineate the underlying disease states. The dosage ranges from 2 to 5 mCi.
6-7. Oncology
Both diagnostic and therapeutic radiopharmaceuticals are available for use in oncology. The diagnostic radio-pharmaceuticals include traditional tracers, a radio-labeled peptide, a monoclonal antibody, and a PET agent. The therapeutic radiopharmaceutical is a radio-labeled monoclonal antibodies.
Fludeoxyglucose F-18 (F-18 FDG)
Fludeoxyglucose is a glucose analog in which F-18 has replaced a hydroxyl group. After the radiolabeled fludeoxyglucose is transported into a cell by facilitated diffusion, it becomes trapped inside the cell after being phosphorylated. Metabolically active cells take up this PET radiopharmaceutical according to their glucose utilization rate, so the more metabolically active (as in cancer), the greater the degree of uptake. Imaging of various neoplasms is performed with a PET scanner. The dosage range for an adult is 10–20 mCi, and the pediatric dosage range is 0.14–0.2 mCi/kg.
In-111 Pentetreotide (OctreoScan)
This tracer is a pentetate conjugate of octreotide, which is radiolabeled with In-111, and it attaches to somatostatin receptors throughout the body. Tumors having a high density of somatostatin receptors will take up this radiolabeled peptide and be demonstrated with scintigraphy. The FDA has approved this radio-pharmaceutical for the scintigraphic localization of primary and metastatic neuroendocrine tumors having somatostatin receptors.
The patient should be well hydrated prior to and after radiopharmaceutical administration; bowel cleansing with a mild laxative is suggested the night before injection of the tracer and continuing for 48 hours. If a patient has an insulinoma, bowel cleansing should be conducted only if the patient’s endocrinologist approves. The recommended adult radiopharmaceutical dosage for use in planar imaging is 3 mCi, while the dosage for SPECT imaging is 6 mCi. The sensitivity of the imaging study may be compromised in patients on therapeutic octreotide acetate.
In-111 Capromab Pendetide (ProstaScint)
In-111 capromab pendetide is a diagnostic radio-labeled murine monoclonal antibody that recognizes an antigen present on prostate epithelium. This particular antigen is found on many primary and metastatic prostate cancer cells. In-111 capromab pendetide is indicated for patients having newly diagnosed biopsy-proven prostate cancer who are considered to have localized cancer, as demonstrated by standard diagnostic studies, but are at high risk of pelvic lymphnode metastases. Also, this diagnostic radiopharmaceutical is indicated in postprostatectomy patients who have a rising prostate-specific antigen level, a negative or equivocal standard metastatic evaluation, and a high clinical suspicion of occult metastatic lesions. Clinical trials with this radiotracer reported an 8% incidence of human anti-mouse antibody levels after a single administration, and this should be considered when performing repeat studies or some immunoassays. The recommended dosage is 5 mCi of In-111 radiolabeled to 0.5 mg of capromab pendetide, and the dose should be filtered before administration with a 5 micron filter.
I-123 Iobenguane (I-123 MIBG, AdreView)
I-123 iobenguane is a diagnostic imaging radiopharmaceutical that is used as an adjunct in the identification of primary or metastatic pheochromocytomas or neuroblastomas. Structurally, iobenguane is similar to norepinephrine and guanethidine and follows the same cellular uptake and pathways as norepinephrine. This radiopharmaceutical is subject to interactions with drugs that reduce norepinephrine uptake, and those drugs should be discontinued for a minimum of five biological half-lives if tolerated by the patient. Any norepinephrine uptake inhibition cannot be overcome by increasing the dosage of I-123 iobenguane. A thyroid-blocking agent is recommended prior to administration of this radiopharmaceutical. The recommended adult dosage is 10 mCi, and the pediatric dosage is based on body weight. This preparation contains benzyl alcohol in a concentration of 10.3 mg/mL; thus, the risks of benzyl alcohol should be considered.
Gallium Citrate Ga-67
Gallium citrate is a diagnostic radiopharmaceutical that has indications in both oncology and infectious and inflammatory diseases. After Ga-67 citrate is intravenously injected, the Ga-67 disassociates from the citrate and becomes bound to plasma transferrin. The exact mechanism of localization of this tracer is not known even though it has been studied at great length. Its oncological indications include identifying the presence and extent of Hodgkin disease, lymphoma, and bronchogenic carcinoma. The dosage range is 3–10 mCi, depending on the disease state and whether planar or SPECT imaging is used.
I-125 Lotrex
It is used to deliver intracavitary radiation therapy in patients with malignant brain tumors following tumor resection surgery. A balloon is surgically placed in the cavity of the removed tumor and filled with the radiopharmaceutical. The radiopharmaceutical, but not the balloon, is removed after the intended radiation treatment is completed. The patient stays in the hospital the entire time the balloon is filled.
Y-90 Ibritumomab Tiuxetan (Y-90 Zevalin)
Y-90 ibritumomab tiuxetan is a CD20-directed radiotherapeutic antibody for patients with relapsed or refractory, low-grade or follicular B-cell non-Hodgkin lymphoma (NHL) or patients with previously untreated follicular NHL who achieved a partial or complete response to first-line chemotherapy. The CD20 antigen is expressed on pre-B-lymphocytes and mature B-lymphocytes and on > 90% of B-cell NHLs.
Ra-223 Dichloride (Xofigo)
It is an alpha particle–emitting radioactive therapeutic agent indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastatic disease. The dose regimen is 1.35 mCi/kg patient body weight, administered at 4 week intervals for a total of six injections. The active moiety of Ra-223 dichloride is the alpha particle–emitting radionuclide radium 223, which mimics calcium and forms complexes with the bone mineral hydroxyapatite at areas of increased bone turnover, such as bone metastases. The high linear energy transfer of alpha emitters (80 keV/micrometer) leads to a high frequency of double-strand DNA (deoxyribonucleic acid) breaks in adjacent cells, resulting in an antitumor effect on bone metastases. The alpha particle range from Ra-223 dichloride is less than 100 micrometers (less than 10 cell diameters), which limits damage to the surrounding normal tissue.
6-8. Pulmonary Imaging
Pulmonary imaging is performed for two purposes, ventilation and perfusion, which are often ordered as a V/Q or V/P scan. Ventilation and perfusion studies will match in some disease states and will not match in others. The results of both imaging procedures help identify different diagnoses.
Ventilation
Xenon (Xe)-133 gas
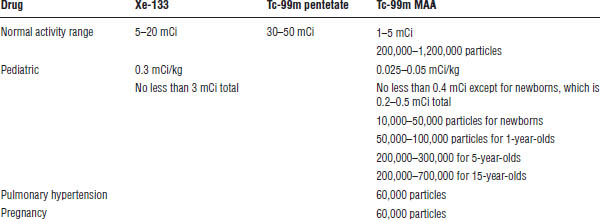
Xe-133 gas is indicated for the diagnostic evaluation of pulmonary function. A side beta emission causes a higher-than-ideal radiation exposure for imaging and may be avoided in pediatrics for this reason. The radiopharmaceutical is administered via inhalation from a closed respirator system with dosage ranges listed in Table 6-3. The patient is asked to inhale and hold his or her breath for a static image and then breathes in fresh air and exhales Xe-133 gas into a collecting bag or trapping machine while images are acquired to determine the washout period. Obstruction in airways will be detected as radioactive after the washout. The advantage of Xe-133 gas is that it is more physiologic than aerosolized Tc-99m DTPA and affords the ability to perform not only a wash-in phase but also a washout phase. Xe-133 is produced by a cyclotron and is manufactured in 10 mCi and 20 mCi vials every week. It typically expires 1 week past calibration date and may be dispensed as early as 1 week precalibration date. The ventilation study is routinely performed before the perfusion study.
Tc-99m pentetate (Tc-99m DTPA)
The kit for the preparation of Tc-99m pentetate injection is used off label as a standard of practice for pulmonary ventilation studies. The radiopharmaceutical is administered via a commercially available nebulizer in 2–3 mL in the dosage ranges listed in Table 6-3. The patient inhales the aerosol and exhales for 5–10 minutes. The higher-than-Xe-133 gas activity is attributable to the fact that less than 5% of the nebulized activity is retained in the lungs. The advantage over Xe-133 gas is the preferable imaging decay characteristics of Tc-99m as well as the ability to image in multiple angles compared with usually just the posterior angle; however, only wash-in images can be obtained. The ventilation study is routinely performed before the perfusion study.
Table 6-3. Radiopharmaceuticals for Pulmonary Imaging
Perfusion
Tc-99m albumin aggregated (Tc-99m MAA)
The kit for the preparation of Tc-99m albumin-aggregated injection is indicated as a lung-imaging agent that may be used as an adjunct in the evaluation of pulmonary perfusion in adult and pediatric patients. Primarily, it is used in the detection of a pulmonary embolism. The radiopharmaceutical is administered intravenously in dosage ranges listed in Table 6-3. An average of 200,000–700,000 macro aggregated albumin particles (10–150 micrometers in diameter) tagged to Tc-99m are injected and occlude pulmonary capillaries in a uniform distribution, assuming the patient is supine when injected. Any areas of decreased activity indicate a decrease in blood flow or blocking of that capillary. Decreasing activity and particle numbers should be considered with pediatric and pregnant patients as well as those with a left-to-right cardiac shunt and with pulmonary hypertension. Severe pulmonary hypertension is a contraindication.
6-9. Renal Imaging
Renal function refers to the glomerular filtration and the tubular secretion in the nephron. In a healthy patient, 20% of the renal plasma flow undergoes filtration and 80% undergoes tubular secretion. Renal scintigraphy can be divided into a few main studies: perfusion, diuresis, angiotensin-converting enzyme (ACE) inhibition, and cortical. Three different drugs play an important and particular role in each of these (see Table 6-4).
Perfusion and Diuresis Studies
Renograms are separated into three different areas: arrival of the radiopharmaceutical, renal accumulation of the radiopharmaceutical, and excretion of the radiopharmaceutical into the urine. The patient should be well hydrated before the study and must void before administration and initiation of the study. Imaging takes place immediately after administration. Functional information is obtained with these studies.
If obstruction needs to be ruled out, a loop diuretic is used to see if washout of activity occurs. If none occurs, an obstruction is suspected.
Tc-99m mertiatide (Tc-99m MAG3)
About 40–50% of the injected drug is extracted by the tubules with each pass through the kidneys, and this drug is extracted 100% by the tubules. The high extraction efficiency makes it ideal for patients with and without renal insufficiency.
Tc-99m pentetate (Tc-99m DTPA)
For this pure glomerular filtration agent, the extraction percentage with each pass through the kidneys of this drug is 10–20% in patients with normal renal function.
ACE Inhibitor Studies
With renal artery stenosis, glomerular filtration rate is maintained because of the pressure exerted by the constricted efferent arteriole but maintains normal Tc-99m DTPA extraction. An hour after the baseline study, an ACE inhibitor is administered, followed by another injection of the radiopharmaceutical. Decreased urine flow caused by administration of ACE inhibitor produces a delayed washout of Tc-99m MAG3, resulting in cortical retention. Whereas when Tc-99m DTPA is the radiopharmaceutical, the ACE inhibitor reduces extraction of the radiopharmaceutical. Care must be taken to know which drug is used in the study to properly interpret the results. Captopril 25 mg or 50 mg is typically administered with the radiopharmaceutical injected 1 hour afterward. Enalaprilat (Vasotec) 0.04 mg/kg (2.5 mg maximum) can also be used with the radio-pharmaceutical injected 15 minutes afterward.
Table 6-4. Radiopharmaceuticals for Renal Imaging

Cortical Studies
Imaging is used to help detect renal infection or scarring, mostly in the diagnoses of acute pyelonephritis. This imaging is primarily performed in pediatric patients.
Tc-99m succimer (DMSA)
Approximately 40–50% of the injected dose is bound in the cortex, and imaging is acquired 1.5–3 hours after administration to ensure maximal uptake and background clearance. Delayed imaging can be performed for up to 24 hours.
6-10. Reticuloendothelial, Hepatobiliary, and Gastrointestinal Systems
The reticuloendothelial system (RES) includes the imaging of the liver Kupffer cells, spleen, and bone marrow. The hepatobiliary system includes the liver, gall bladder, and bile ducts. The gastrointestinal system includes the mouth, esophagus, stomach, and small and large intestines.
RES Imaging
RES imaging is based on the use of radiocolloids (Tc-99m sulfur colloid) to take advantage of the Kupffer cells for liver imaging. Distribution of the drug is based on blood flow, presence of any disease states, and the particle size and numbers administered. The anatomy of the RES can be imaged as early as 10 minutes after administration, and function can be determined as well. For instance, liver disease and tumors will decrease or restrict blood flow to certain areas, creating photopenic areas on a scintigraphic image. When cells are damaged by disease or other means, such as radiation or other trauma, Tc-99m sulfur colloid is not taken up and the drug is shunted to other areas. Accumulation in a healthy patient is 80–85% in the liver, 5–10% in the spleen, and the rest in the bone marrow via adventitial cells; the smaller particles preferentially are accumulated in the bone marrow and larger particles preferentially in the liver. A greater number of particles facilitates with bone marrow imaging.
Tc-99m sulfur colloid
Imaging can begin within 20 minutes of administration. Particles range in size from 1.5 micrometers to less, depending on compounding parameters. The drug can be filtered through a 0.1 or 0.22 micrometer filter to reduce particle sizes and help enhance bone marrow imaging.
Technetium sulfur colloid contains only inorganic sulfur and is completely void of any sulfhydryl or disulfide groups, and as such is not contraindicated in patients with a known allergy to sulfa drugs.
Hepatobiliary System
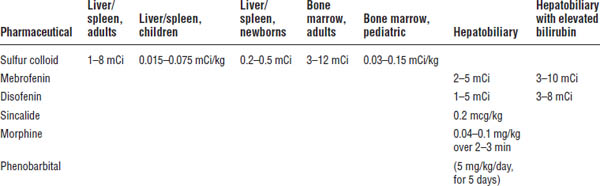
Also known as HIDA scans, these radiopharmaceuticals have the same hepatocyte uptake, transport, and excretion pathways as bilirubin, which means they are extracted by the hepatocyte, secreted into the bile canaliculi, and cleared through the biliary tract into the bowel. As such, the bilirubin level in a patient can interfere with the study, and larger dosages of radioactivity must be administered to compensate (Table 6-5). Clinical diagnoses that can be evaluated with this procedure are listed in Box 6-1.
Tc-99m mebrofenin (Choletec)
This kit contains methylparaben and propylparaben, giving it a beyond-use date of 18 hours. It is less sensitive to bilirubin interaction than is disofenin.
Tc-99m disofenin (Hepatolite)
The compounded kit has a beyond-use date of 6 hours and is more sensitive to bilirubin interaction than is mebrofenin.
Sincalide (Kinevac)
Sincalide is a nonradioactive cholecystopancreatic-gastrointestinal hormone peptide for parenteral administration that constricts the gall bladder. It can be used to establish whether a complete or near-complete obstruction of the common bile duct exists, to obtain the gall bladder ejection fraction, or to increase diagnostic ability to detect acute cholecystitis with a prolonged fasting patient.
Morphine
Morphine can be used to help evaluate cystic duct obstruction by constricting the sphincter of Oddi and restricting the radiopharmaceutical from entering the duodenum and forcing the flow into the gallbladder if the cystic duct is patent.
Table 6-5. Radiopharmaceuticals for Hepatobiliary Imaging
Phenobarbital
Pretreatment (5 days) with phenobarbital can be used to detect biliary atresia and to differentiate it from other causes of neonatal jaundice. It maximizes sensitivity by activating the liver excretory enzymes, and the lack of any biliary clearance into the bowel within 24 hours indicates a true positive test.
Gastrointestinal System
Solid gastric emptying and motility studies are performed using Tc-99m sulfur colloid compounded into liquid egg whites, whole eggs, and other various foods. The patient should be fasting for 4 hours before the procedure and should be off any medications that are prokinetic or delay gastric emptying (see Table 6-6 and Box 6-2). Transit times should be compared to local institutional baselines from the same delivery meal. Testing for gastroesophageal reflux disease is also available using Tc-99m sulfur colloid or In-111 DTPA compounded into a liquid solution such as milk (Table 6-7). This can also be used to test for the liquid phase of gastric emptying.
Box 6-1. Clinical Indications for Hepatobiliary Imaging
Acute cholecystitis
Chronic cholecystitis
Common duct obstruction
Choledochal cyst
Biliary atresia
Postoperative biliary tract
Liver transplants
Trauma
Primary benign and malignant tumors
Enterogastric bile reflux
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


