Normal Ovaries, Inflammatory and Non-Neoplastic Conditions
Anatomy, Histology, and Function
Anatomy
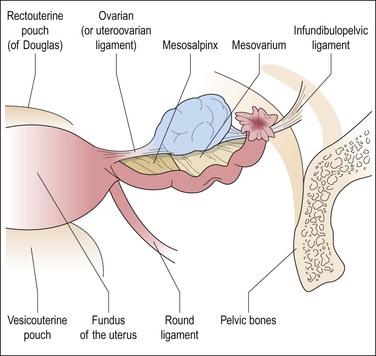
Each ovary is attached along its hilar aspect to the broad ligament by a double fold of peritoneum, the mesovarium (Figure 23.1).1 The ovarian ligament attaches the ovarian medial pole to the uterine cornu just below and behind the fallopian tube. The lateral pole of the ovary is attached to the pelvic wall by the suspensory ligament (infundibulopelvic ligament), a peritoneal fold containing the principal vascular supply and lymphatic drainage of the ovary. Veins from the left ovary drain into the left renal vein. Those of the right ovary drain into the right renal vein in 10% of women and directly into the inferior vena cava in 90%.2
Histology
The ovary is covered by a single layer of modified mesothelium originating from the celomic epithelium. This highly specialized mesothelial layer is continuous with the mesothelium of the peritoneal cavity at the ovarian hilus. Previously referred to as the ‘germinal epithelium,’ this layer is more appropriately designated as the ‘surface epithelium.’ The cells vary from columnar to cuboidal to flat (Figure 23.2). Immunohistochemically, the surface epithelial cells react for cytokeratin (CK)7, vimentin, Ber-EP4, N-cadherin, and calretinin,3 but generally lack reactivity for CK20, desmin, and CA125. These cells are more active than the peritoneal mesothelial cells to which they are related and secrete or have receptors for inhibin, estrogen, progesterone, and androgens, as well as a range of growth factors and cytokines, such as epidermal growth factor, transforming growth factor-β (TGF-β), and tumor necrosis factor-α (TNF-α).4 They form a delicate layer that is often partially denuded as a result of handling at surgery. The surface epithelium often forms invaginations and inclusion cysts within the ovarian stroma. The ovarian surface epithelium is discussed further in relation to the histogenesis of epithelial tumors in both the ovary and the peritoneum (see Chapters 25 and 31, respectively).
Figure 23.2 Cuboidal surface epithelial cells covering the ovary of a perimenopausal patient. They are separated from the underlying stroma by a distinct basement membrane.
Physiology
Folliculogenesis
The primordial germ cells arise from the yolk sac endoderm and migrate via the hindgut mesentery to the gonadal ridge, the migration commencing 3 weeks after fertilization.5 At this stage the proliferating germ cells constitute the dominant growth, forming a finite complement of about six million ova by the sixth month of fetal life, to which there is no later addition. This proliferation is evident by the 8th week and is marked by the 13th week when the ovary consists largely of oogonial and oocytic clusters that taper proximally, with ‘cords’ only in the rete. Pregranulosa cells enclose individual germ cells to form primordial follicles (Figure 23.3A). At the primary oocyte stage, the cells are in the prophase of the first meiotic division and DNA synthesis resumes only in the event of fertilization. At birth, the ovary consists largely of scanty, loose stroma, enclosing primordial and maturing follicles and folded around stems of vascular connective tissue. The characteristic ovarian stroma develops during the first year. The total number of germ cells at birth exhibits a considerable variation around an approximate average of 500,000, already a significant loss from the second trimester maximum.
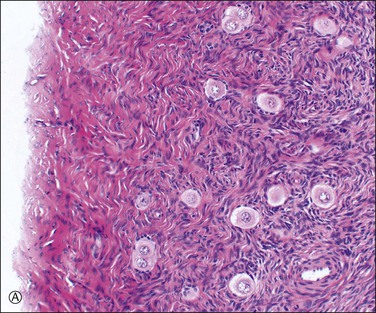
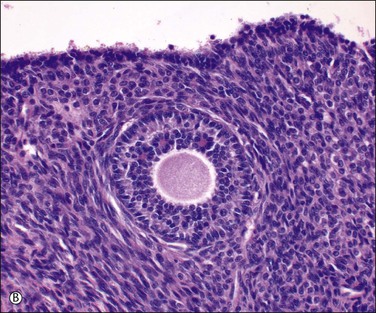
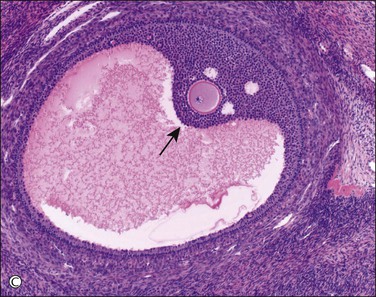
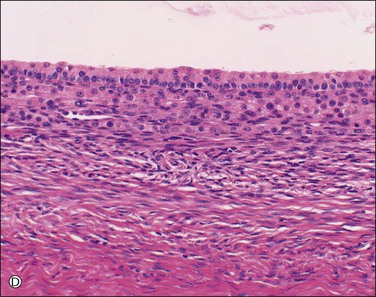
Figure 23.3 Preovulatory follicles. (A) Cellular superficial cortex containing a number of primordial follicles. An unusual variation is the presence of binovular follicles. (B) A late primary or preantral follicle with a prominent zona pellucida surrounding the ovum and an occasional Call–Exner body within the developing granulosa cell layer (above the ovum). The spindle-shaped cells of the emerging theca are apparent. A regressing cystic follicle forms the upper margin of the field. (C) Secondary or graafian follicle with an eccentric cumulus oophorus containing the oocyte (arrow). (D) Developing follicle showing the granulosa cell layer subtended by theca interna (plump cells) and theca externa (spindle cells) layers.
Follicular Maturation
Typically, a single dominant follicle develops, resulting in a single ovulation during each cycle. During the early follicular phase selection of a dominant follicle takes place. This complex process involves insulin-like growth factors and locally produced substances.6–8 The early rise of FSH levels helps recruit the dominant follicle. The transient decrease in FSH during the midfollicular phase prevents development of subdominant follicles. The largest follicle has more granulosa cells and therefore more FSH receptors, enabling its continued growth despite the low FSH levels.9 Moreover, the dominant follicle’s granulosa cells are not only sensitized to FSH but at this stage express LH receptors and therefore respond directly to LH as well as FSH.10,11
Follicular maturation comprises enlargement of the ovum, formation of the zona pellucida around it, proliferation of the granulosa cells, and formation of a cavity among the latter. The enlarging follicle expands gradually. Several months are required for a new growing follicle to reach the preantral stage (0.15 mm), and an additional 70 days to reach a 2 mm size. It is likely that several hundred primordial follicles initiate growth each month, but only 20 or so become ‘precursor’ (preovulatory) follicles. From the time they enter the selectable stage during the late luteal phase, preovulatory follicles become sensitive to cyclic changes of FSH in terms of granulosa cell proliferation. From the midfollicular phase, the preovulatory follicle synthesizes high quantities of estradiol, and, after the midcycle gonadotropin surge, very large amounts of progesterone. At this stage, the follicle is maximally responsive to gonadotropins, and especially to LH, which triggers granulosa wall dissociation and cumulus expansion as well as oocyte nuclear maturation. At this phase, the follicle is about 2–5 mm in diameter, with a fluid-filled antrum, more than one million granulosa cells, and a single oocyte. The other follicles undergo atresia at an early stage of development. Thus, as the follicle develops, its responsiveness to gonadotropins progressively increases under the control of local factors acting in an autocrine/paracrine fashion.12 In addition to the critical endocrine signaling pathways between the hypothalamus, pituitary, and ovary, it is now evident that the proteins of the TGF-β superfamily secreted by the oocyte itself are important in influencing the microenvironment of the developing follicle.13
Granulosa cell proliferation begins when the ovum starts to enlarge. The cells change from flat to cuboidal and then form several layers around the ovum (Figure 23.3B). Small periodic acid–Schiff (PAS)-positive droplets form between the granulosa cells and condense around the ovum to form small spaces. Enlargement or fusion of these spaces produces first an arcuate crevice and then a reniform cavity, the antrum (Figure 23.3C). The granulosa cells form a palisade at the periphery of the follicle and in turn are surrounded by a pale layer of specialized ovarian stroma, the theca interna. A basal lamina separates the granulosa cells from the cells of the theca interna. The theca externa is a poorly defined zone formed by the merging of the theca interna with the surrounding ovarian stroma (Figure 23.3D). Theca cells are fundamental for follicular growth, synthesizing all androgens required by the developing follicles for conversion into estrogens by the granulosa cells, and providing structural support of the growing follicle as it progresses through the developmental stages. Theca cells are likely recruited from surrounding stromal tissue by factors secreted from an activated primary follicle.14 During maturation, the cytoplasm of the cells comprising the theca interna accumulates lipids, increases in amount, and becomes foamy or granular. In the dominant preovulatory follicle, the theca cell layer is well supplied with blood vessels but the granulosa cell layer remains avascular.
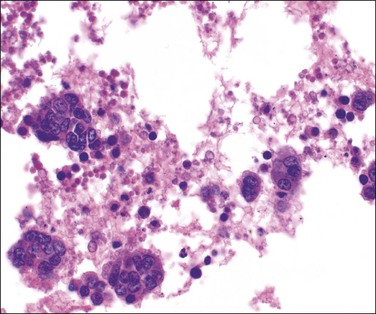
The follicular fluid is a modified transudate from the theca and adjacent vessels, which are believed to become more permeable because of locally released histamine. The same transudative process leads to the development of a loose-textured vascular shell around the outside of the follicle, the theca externa. As the antrum enlarges, the granulosa cell layer generally thins, but persists as a mound, the cumulus oophorus, around the ovum (Figure 23.3C). Granulosa cells aspirated from the developing follicle at this stage tend to be single or in small clusters with scanty amphophilic cytoplasm (Figure 23.4). Outside the cumulus the theca interna shows a corresponding thickening, the theca cone, which has a role in loosening the coherence of the tissues. The mature, preovulatory follicle is known as a graafian follicle. The final phase in follicular maturation is abrupt and largely mediated by the LH surge and a complex interaction of local cytokines.15 It includes rapid accumulation of follicular fluid, loosening of the theca, and discharge of the fluid contents and ovum with the few attendant granulosa cells that form the corona radiata. The follicle then collapses, taking on a corrugated outline that becomes exaggerated as transformation into a corpus luteum advances. Bleeding into the thecal tissues may occur at about the time of ovulation but, as the granulosa is as yet avascular, there is normally no bleeding into the follicular cavity and peritoneum.
Formation of the Corpus Luteum
The formation of the corpus luteum comprises two processes: (1) blood vessels grow into the collapsed granulosa cell layer from the theca, carrying theca cells with them (some bleeding now takes place into the cavity and, because of this, the corpus luteum of the current cycle appears bluish when seen through the surface of the ovary); and (2) the cells comprising the granulosa cell layer luteinize (Figure 23.5), the cells becoming strikingly large and pale, and readily distinguishable from the smaller and somewhat less pale luteinized theca cells (‘theca–lutein cells’). Ultrastructural studies of the luteinized cells show abundant smooth endoplasmic reticulum with vesicular dilatations, mitochondria with tubular cristae, and numerous small lipid droplets, which are the familiar general features of the steroid-secreting cell.
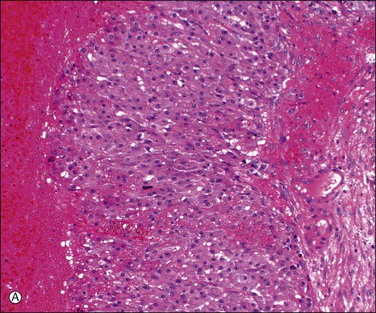
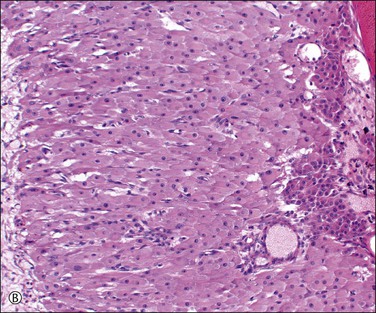
Figure 23.5 Corpus luteum. (A) Early corpus luteum with antrum to the left, granulosa lutein layer in the center, and poorly formed theca to the right but no vascularization of the granulosa. (B) Mature corpus luteum with vascularized granulosa and immature granulation tissue organizing the central coagulum (right).
When pregnancy occurs, the corpus luteum remains unchanged for about 50 days. It then doubles in size during the following 10 days and remains thus enlarged until about day 80, when it begins to shrink as the placenta takes over progesterone production. Hyaline bodies, about 10–20 µm in size, are found sparingly among the luteinized cells only in the corpus luteum of pregnancy (Figure 23.6A). Another histologic feature that, when present, distinguishes the corpus luteum of pregnancy from that of menstruation is the organization of the central coagulum to produce mature, avascular, collagenous connective tissue over a time period not available to the corpus luteum of menstruation prior to its programmed involution (Figure 23.6B).
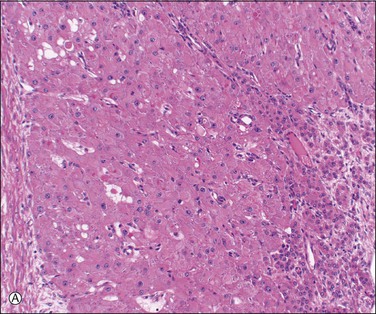
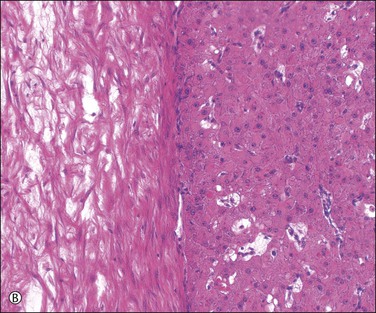
Figure 23.6 Corpus luteum of pregnancy. (A) Established corpus luteum of pregnancy with organizing granulation tissue lining antrum to the right, granulosa lutein layer in the center, containing scattered hyaline bodies, and theca to the left. (B) Active corpus luteum of pregnancy with avascular collagenous connective tissue of many weeks’ age.
Involution of the Corpus Luteum
Unless stimulated by β-human chorionic gonadotropin (β-hCG), produced by the trophoblast of the fertilized zygote, the corpus luteum begins its programmed involution at about day 9, i.e., about day 23 of a normal 28 day cycle. The biologic mechanisms responsible for this physiologic destructive process of the corpus luteum (structural luteolysis) are complex and centered on apoptosis.16,17 Hyaline and fatty changes appear to a variable extent in the luteinized granulosa cells (Figure 23.7A), together with an increase of acid phosphatase and PAS-positive intracellular material. This apoptotic change may occur acutely, with frank necrosis (Figure 23.7B). The average corpus luteum reaches about 1.5 cm in maximum size. It has shrunk only slightly by the end of the cycle, but then rapidly gets smaller. As it does so, its wavy lemon-yellow form becomes thinner and the color deepens to a darker yellow or orange. Eventually, the luteal pigment and the cells disappear. After several months, the corpus luteum completes its transformation into a much folded, collapsed shell of white, hyaline collagen, the corpus albicans (Figure 23.7C). Corpora albicantia persist in the medulla and are often conspicuous on the cut surface, particularly in later life. Regression of the corpus luteum of pregnancy is occasionally accompanied by residual microcalcispherule formation (Figure 23.7D).
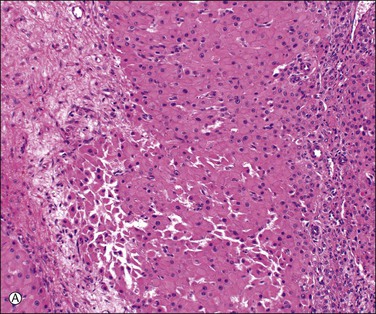
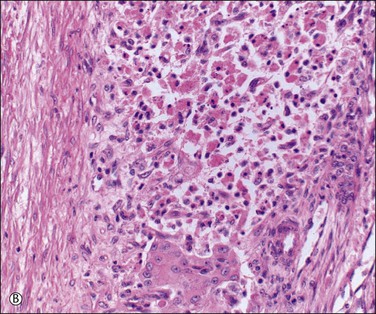
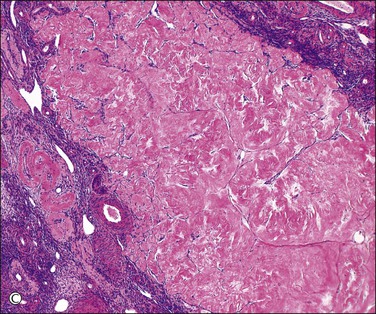
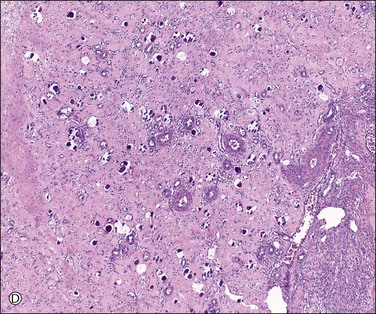
Figure 23.7 Regression of corpus luteum. (A) Regressing corpus luteum, showing considerable reduction in size of luteal cells (early apoptosis) and complete vascularization of the granulosa. (B) Hyperacute regression (diffuse apoptosis) of a corpus luteum, with frank necrosis. Surviving granulosa cells at the bottom. (C) Corpus albicans. (D) Regressed corpus luteum of pregnancy with prominent microcalcispherules.
Follicular Atresia
During each cycle many follicles start to mature but usually only one ruptures to form a corpus luteum. Occasionally, two or three synchronous corpora lutea are found (one mechanism of multiple pregnancies). The follicles that do not complete maturation become atretic. In atretic antral follicles, granulosa cells stop proliferating and become apoptotic. Atresia may start at any stage during maturation and proceeds at rates varying from follicle to follicle and in different parts of the same follicle.18,19 Atresia is triggered by a lack of some essential factors supporting follicular development. Specifically, terminal follicular development is strictly dependent upon gonadotropin supply (FSH, then LH in the final preovulatory stage). In addition, paracrine factors (growth factors, CKs, steroids, constituents of extracellular matrix) also play important roles in amplifying gonadotropin action in follicular cells.20 The features of atresia include degeneration and shrinkage of the ovum; apoptosis of the granulosa cells or their transformation into connective tissue cells (Figure 23.8A); reversion of the cells of the theca interna to their original stromal form; invasion and obliteration of the shrinking cavity by stromal cells; and the formation of a hyalinized, collagenous membrane at the margin of the follicle. Eventually, the hyaline membrane alone persists to mark the site of the former follicle, which now appears as a compact white or gray nodule with a wavy outline, the corpus fibrosum (Figure 23.8B).
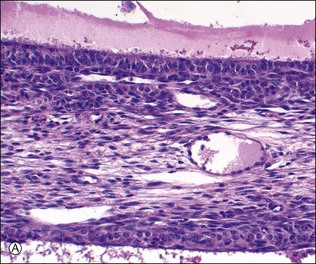
Figure 23.8 Follicular atresia. (A) Adjacent cystic follicles with early atresia in the lower follicle. The granulosa is degenerate and has been partly shed into the antrum. (B) Small follicle showing advanced atresia with typical festooned contour and fibroblastic tissue occupying the site of the original antrum. (C) Atretic follicle in early pregnancy displaying partial luteinization of the theca interna layer and central persistence of small cords of granulosa cells.
During pregnancy, follicle maturation and atresia diminish in the first 10 weeks. They then become more active and are associated with marked theca-cell luteinization (Figure 23.8C).The cellular stroma left after follicular atresia contributes to production of the cellular stroma that typifies the mature ovary and is absent at birth. Stroma formation depends largely on follicular activity and is depressed if follicles are lacking or inactive and increased if maturation and atresia are excessive, as in polycystic ovary syndrome. The medullary and juxtahilar stroma thus has a direct ‘follicular’ ancestry, which makes it easier to understand why the tumors that may rarely arise from it are similar to those that develop in the functioning cortical stroma, i.e., tumors of sex cord–stromal type.
The Hormonal Background of the Ovarian Cycle
The normal menstrual (ovarian) cycle is depicted in Figure 23.9. Increasing levels of FSH stimulation are required for follicular growth.21 Follicular antrum formation and antral expansion are absolutely dependent on pituitary FSH to which the larger follicles are more responsive. Early antral follicles (≤5 mm) develop normally even in infant ovaries but preovulatory (≥20 mm) growth depends on the adult FSH levels reached in ovulatory menstrual cycles and on the follicle capacity for estrogen synthesis. Follicular estrogens feeding back to the hypothalamic–pituitary axis control gonadotropin secretion, the secretion of FSH showing peaks on about days 4 and 14. Local interaction between estrogens and gonadotropins helps to coordinate preovulatory follicular maturation. Signaling by the gonadal paracrine activin and inhibin, which are members of the TGF-β superfamily, is crucial for follicular and oocyte development.22,23 Immature antral follicles produce activin that enhances granulosa cell sensitivity and responsiveness to FSH, thus promoting follicular development, and simultaneously suppresses thecal androgen synthesis. Appropriate stimulation by FSH diverts the follicle to formation of inhibin, which suppresses the secretion of FSH and promotes androgen synthesis by LH-stimulated theca cells. Androgens in turn synergize with FSH to augment inhibin synthesis.
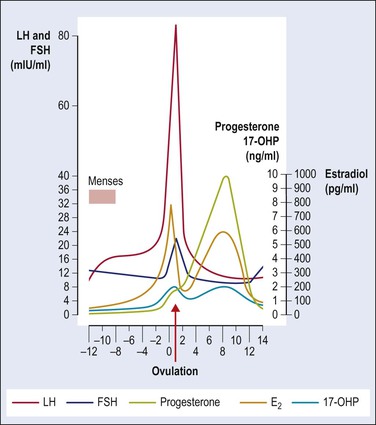
Figure 23.9 Normal menstrual cycle: plasma hormone levels. Ovulation occurs just after LH peak; 17-OHP, 17-α-hydroxyprogesterone. (Reproduced with permission from Prat, 2004.1)
LH acts on specific receptors on theca cells to stimulate androgen production, with positive correlations between androgen receptor expression and follicular cell proliferation.15 Furthermore, androgens are active through a conversion to estrogens in granulosa cells. The acute follicular enlargement leading to ovulation is associated with the peak of LH secretion on day 14 of the normal 28 day cycle, triggered by estrogen secreted by the dominant follicle. If pregnancy does not ensue, the corpus luteum’s life span is maximally about 14 days. As mentioned previously, its degeneration begins by day 23 (day 9 postovulation). Its persistence during that time depends on the continuous low level of LH secretion, but on no other known luteotrophic stimulus. When pregnancy occurs, chorionic gonadotropin fosters maintenance of the corpus luteum.
The levels of LH, FSH, and estradiol fall after ovulation, but there is sufficient LH to maintain the sensitive luteinized granulosa cells of the corpus luteum. Together the luteinized theca cells and luteinized granulosa cells of the corpus luteum produce both estradiol and its major product progesterone. Normal corpus luteum function depends on numerous regulatory factors, such as prostaglandins, oxytocin, steroids, growth factors, cytokines, and so forth.24 The theca cells produce primarily estrogen and the luteinized granulosa cells produce mainly progesterone, which is reflected in the high plasma progesterone level during this phase of the cycle and the high pregnanediol excretion from the time of ovulation until the day or two preceding menstruation. Both hormones reach their peak in the midsecretory phase of the cycle, when estradiol levels are similar to those of the mid to late follicular phase. The stromal tissue produces mainly androstenedione, which has about one-tenth the androgenic activity of testosterone and is partly converted into the latter peripherally. The effects of these hormones on the pituitary are largely mediated through the hypothalamus. Secretion of FSH is inhibited by sufficient amounts of either estrogens or progesterone; the effect of estrogens on LH secretion is thought to be biphasic, and is stimulatory over a short length of time and inhibitory in the long term.
The Ovarian Hilum and Its Vicinity
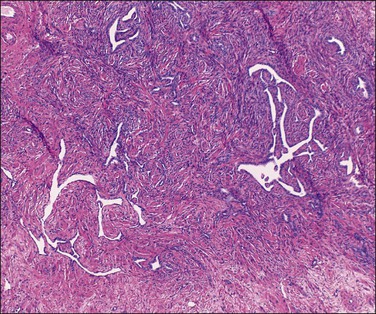
The rete ovarii consists of multiple intercommunicating channels lined by deeply staining, cuboidal to flattened epithelial cells exhibiting a characteristically angular, jagged-looking cross section (Figure 23.10). Nearer to the ovary the rete tubules are more rounded in outline and in this situation they often show traces of a muscle and connective tissue covering. The origin of the rete ovarii is disputed. It is generally considered as homologous with the straight seminiferous tubules of the testis. The relevant immunoprofile of the rete tubules includes strong reactivity for CD1025 and nonreactivity for calretinin.3
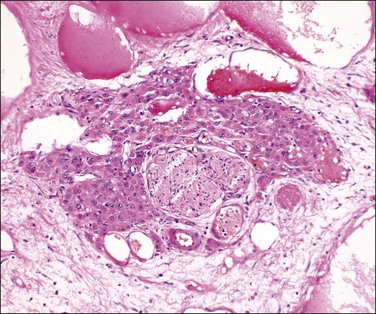
Small groups of lutein-like cells are found in the mesovarium and in the ovarian hilum, often adjacent to nerves. These are the so-called ‘ovarian hilar’ (hilum or hilus) cells (Figure 23.11). They resemble the interstitial cells of the testis (Leydig cells) and contain lipofuscin pigment and Reinke crystals. They produce both androgens and estrogens and express α-inhibin and calretinin.3
Follicular Failure
Follicular Dysgenesis (Dysplasia)
Definition
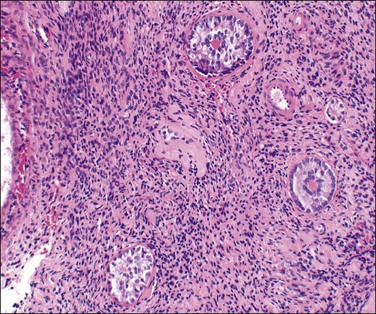
Dysplastic primary (preantral) and secondary (antral) follicles are occasionally encountered in otherwise normally functioning ovaries. The term ‘follicular dysgenesis’ has been applied to polyovular primordial follicles (i.e., containing two or more oocytes; see Figure 23.3A). However, we believe it is most appropriately applied to developing follicles that differ morphologically from normal by disorganized proliferated granulosa, frequently without an antrum despite the follicular size, and containing multiple Call–Exner bodies (Figure 23.12). Microscopically, these lesions have been likened to gonadoblastoma. They occur frequently (25%) in the ovaries of infants and young children. A similar morphologic pattern is seen in some cases of documented hypergonadotropic premature ovarian failure (POF) (Figures 23.12 and 23.13), but the relationship of the follicular changes to altered hypothalamic–pituitary–ovarian function remains unclear. Dysplastic follicles also occur in cases of autoimmune oophoritis and ovarian fibromatosis (see later).
Figure 23.12 Dysplastic follicles in partial resistant ovary syndrome. Bizarre-shaped follicles with normal primordial follicles at top left.
Microscopic Features
The follicular outline is distorted with irregular extensions into the adjacent stroma (Figure 23.12). Sometimes follicular fragmentation produces small nests or cords of granulosa cells. Mitoses are reduced in these cells. Rarely, the cells contain cytoplasmic lipid. A prominent cuff of focally or partially luteinized theca interna cells surrounds about one-third of dysplastic follicles. Atresia of the dysplastic follicles is generally accompanied by persistent granulosa, a phenomenon only occasionally observed in normal ovaries. Occasional dysplastic follicles show convoluted and rounded deposits of hyaline PAS-positive material similar to, and continuous with, the basement membrane (glassy membrane) of the regressing follicles. These deposits are interposed between the cords of granulosa cells in a manner resembling sex cord tumors with annular tubules (SCTAT).
Ovarian Failure
Definition
Failure of the ovary to ovulate or even to produce its steroids may result from multiple factors, ranging from never functioning or functioning abnormally from the start, or more commonly failing at some later time in life.
Ovarian failure may be associated with either elevated or depressed gonadotropin levels:
• Hypergonadotropic hypogonadism (primary ovarian failure) results from a congenital or acquired intrinsic ovarian defect, or failure of the ovaries to respond to pituitary gonadotropins despite the presence of follicles.
• Hypogonadotropic hypogonadism is due to failure of the pituitary gland to secrete gonadotropins (FSH and LH) in amounts sufficient to stimulate the ovaries.
Both primary ovarian failure and hypogonadotropic hypogonadism can present as primary or secondary amenorrhea. The median age at menopause in Western populations of women is approximately 51 years. While about 5% of women experience ‘early’ cessation of ovarian function at or prior to age 45, 1% of women experience POF, classically defined as 4–6 months of amenorrhea under the age of 40 years associated with menopausal levels of serum gonadotropins (FSH > 30U/I) and hypoestrogenism.26 It represents a major cause of female infertility and renders remarkable impact on women’s health due to premature osteoporosis, vaginal dryness, increased risk for cardiovascular disease, and shortened life span.27–29 The human ovary is endowed at birth with a fixed number of primordial follicles, which steadily decreases throughout life due to atresia and recruitment for ovulation.30 A causal relationship between follicular depletion and normal menopause clearly exists and there is a gradual acceleration toward follicular wastage, commencing more than a decade before cessation of menstrual activity. This suggests that follicle dynamics rather than neuroendocrine function determines the onset of menopause, both normal and premature.
POF is most commonly idiopathic,27 but four broad etiologic categories are known:31–33
1. Genetic. Clear-cut X chromosome deletions or transpositions,34–36 particularly those in the critical region, result in a truncated reproductive life span. Autosomal anomalies are less frequent causes.37
2. Autoimmune. POF may accompany any autoimmune polyglandular failure syndrome, most often in linkage with antithyroid and antiadrenal antibodies.38,39
3. Iatrogenic. Repeated ovarian surgery, uterine vascular embolization,40 the underlying disease that led to surgery, or chemotherapy may trigger the failure.41
4. Environmental. Environmental toxins are difficult to pinpoint but, among others, industrial toxins42,43 and galactose consumption44 have been implicated.
Clinical Features
Ovarian failure affects approximately 1 in 10,000 women by age 20; 1 in 1000 women by age 30; 1 in 100 women by age 40.45 Ovarian failure divides into two broad clinicopathologic subtypes: (1) premature follicular depletion, which has been recently termed primary ovarian insufficiency,46 a state of irreversible ovarian failure due to depleted follicles (afollicular ovarian failure), and (2) the much rarer resistant ovary syndrome (‘gonadotropin insensitivity syndrome’), in which primordial follicles are present but fail to respond appropriately to gonadotropin stimulation (follicular ovarian failure). This separation into afollicular and follicular forms is somewhat artificial, since many of the former are very likely end stages of various disorders. This distinction is nonetheless useful clinically, since premature follicular depletion signifies permanent loss of reproductive function.
Although ovarian biopsy, currently by minilaparoscopic techniques through a 2 mm port,47 is not necessary for the diagnosis of ovarian failure, it is the best means to distinguish afollicular from follicular types and is therefore useful in counseling patients desiring pregnancy.48 Recent research suggests that serum anti-müllerian hormone (AMH) levels can differentiate follicular from afollicular patients.49,50 AMH is produced by the granulosa cells of early developing follicles, therefore serum AMH level is proportional to the number of small antral follicles; it is decreased with age and undetectable in the postmenopausal period.51,52
A combined pathologic and etiologic classification of ovarian failure is given in Table 23.1.
Afollicular Ovarian Failure (Primary Ovarian Insufficiency, Premature Follicular Depletion)
Etiology
In most cases, the etiology is unknown.53 Genetic abnormalities more commonly present with primary rather than secondary amenorrhea, and include perturbations of the X chromosomes27,34,36,45,46,54,55 as well as autosomal defects. Normal ovarian function requires two functioning X chromosomes.28 The most obvious genetic cause of primary afollicular ovarian failure is Turner’s syndrome, with complete or near complete loss of the second X chromosome.28 Other well-documented causes of primary afollicular ovarian failure are mixed gonadal dysgenesis (Swyer syndrome; 46,XY), and so-called ‘pure gonadal dysgenesis’ (46,XX).56 Additional reported associations include 47,XXX,57 48,XXXX,58 45,X/46,XX mosaicism,59,60 structurally abnormal X chromosomes,61 and balanced62 and unbalanced63 translocations of the X chromosome, including deletions of the distal portions of the Xq encompassing the fragile X locus.64 The fragile X syndrome results from hypermethylation of the FMR1 gene causing the gene to be transcriptionally silenced. Recent reports of premutation-specific phenotypes unrelated to fragile X syndrome have shown up to 20-fold increased risks for POF among female carriers.65,66
About 10% of patients with POF have a family member with the same condition and a higher percentage if presenting during their teenage years. Some have a known specific genetic abnormality such as galactosemia. The mechanism of ovarian damage in galactosemia remains unknown, but appears to be independent of dietary management and most investigators favor a toxic effect of galactose or its metabolites on germ cells/follicular structures.44,67,68 Ovarian failure occurs in females with the blepharophimosis, ptosis, and epicanthus inversus syndrome (BPES), a human disorder caused by mutations in the transcription factor gene FOXL2 of chromosome 3 and shows facial dysmorphism,69 and rarely in association with the Malouf syndrome,70 Perrault syndrome,71 Roberts-SC phocomelia syndrome,72 noggin mutation,73,74 cutis marmorata telangiectatica congenita syndrome,75 Mulibrey nanism,76,77 and specific types of leukodystrophy.78
Although the etiology remains elusive in most cases with a normal 46,XX karyotype, ovarian autoimmune reaction occurs in 4–5% of women with ovarian insufficiency.79 Clinical gonadal failure is found in both type I and type II autoimmune polyendocrine syndromes.12,80 These include Addison’s,81 Grave’s, and Hashimoto’s diseases, rheumatoid arthritis,82 myasthenia gravis,83 diabetes mellitus,84 pernicious anemia,85 hypoparathyroidism,85,86 and systemic lupus erythematosus,87 ranging, in some studies, in up to 20–40% of patients with POF.
Further support lies in finding antiovarian antibodies in many POF patients (both with and without associated autoimmune disorders), and reports of response to corticosteroid therapy. Ovarian antibodies occur at a higher frequency in infertile women than in the general female population.88 Antibodies in patients’ sera have reacted against steroid-producing cells, gonadotropins and their receptors, corpus luteum, zona pellucida, and oocytes,89 as well as producing a cytotoxic effect on granulosa cells in culture. Because of the association with other autoimmune diseases, investigation and close follow-up are recommended in such patients with POF.79
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree