INTRODUCTION
The term “normal microbial flora” denotes the population of microorganisms that inhabit the skin and mucous membranes of healthy normal persons. The microorganisms that live inside and on humans (now referred to as the normal microbiota) are estimated to outnumber human somatic and germ cells by a factor of 10. The genomes of these microbial symbionts are collectively defined as the microbiome. Research has shown that the “normal microbiota” provides a first line of defense against microbial pathogens, assist in digestion, play a role in toxin degradation, and contribute to maturation of the immune system. Shifts in the normal microbiota or stimulation of inflammation by these commensals may cause diseases such as bacterial vaginosis, periodontitis, and inflammatory bowel disease.
HUMAN MICROBIOME PROJECT
In a broad attempt to understand the role played by resident microbial ecosystems in human health and disease, in 2007, the National Institutes of Health launched the Human Microbiome Project. One of the main goals of this project is to understand the range of human genetic and physiologic diversity, the microbiome, and the factors that influence the distribution and evolution of the constituent microorganisms. One aspect of this project involves having several research groups simultaneously embark upon surveying the microbial communities on human skin and in mucosal areas such as the mouth, esophagus, stomach, colon, and vagina using small-subunit (16S) ribosomal RNA gene sequencing. Among the questions that will be addressed by this project are: How stable and resilient is an individual’s microbiota throughout one day and during his or her lifespan? How similar are the microbiomes between members of a family or members of a community or across communities in different environments? Do all humans have an identifiable “core” microbiome, and if so, how is it acquired and transmitted? What affects the genetic diversity of the microbiome, and how does this diversity affect adaptation by the microorganisms and the host to markedly different lifestyles and to various physiological or pathophysiological states? Numerous observations have already been made. For example, it has been determined that there are large differences among individuals in terms of the numbers and types of species of microorganisms inhabiting the colon and that obesity may be correlated with the types of microbes involved in specific metabolic pathways in the gastrointestinal tract. Readers should be aware that this field is rapidly evolving, and our understanding of the human microbiota will necessarily change as more information about resident microbial communities becomes available through the Human Microbiome Project.
ROLE OF THE RESIDENT MICROBIOTA
The skin and mucous membranes always harbor a variety of microorganisms that can be arranged into two groups: (1) the resident microbiota consists of relatively fixed types of microorganisms regularly found in a given area at a given age; if disturbed, it promptly reestablishes itself; and (2) the transient microbiota consists of nonpathogenic or potentially pathogenic microorganisms that inhabit the skin or mucous membranes for hours, days, or weeks. The transient microbiota is derived from the environment, does not produce disease, and does not establish itself permanently on the surface. Members of the transient microbiota are generally of little significance so long as the normal resident flora remains intact. However, if the resident microbiota is disturbed, transient microorganisms may colonize, proliferate, and produce disease.
Organisms frequently encountered in specimens obtained from various areas of the human body—and considered normal microbiota—are listed in Table 10-1. The classification of anaerobic normal bacterial flora is discussed in Chapter 21.
Skin Staphylococcus epidermidis Staphylococcus aureus (in small numbers) Micrococcus species α-Hemolytic and nonhemolytic streptococci (eg, Streptococcus mitis) Corynebacterium species Propionibacterium species Peptostreptococcus species Acinetobacter species Small numbers of other organisms (Candida species, Pseudomonas aeruginosa, etc) |
Nasopharynx Any amount of the following: diphtheroids, nonpathogenic Neisseria species, α-hemolytic streptococci, S epidermidis, nonhemolytic streptococci, anaerobes (too many species to list; varying amounts of Prevotella species, anaerobic cocci, Fusobacterium species, etc) Lesser amounts of the following when accompanied by organisms listed above: yeasts, Haemophilus species, pneumococci, S aureus, gram-negative rods, Neisseria meningitidis |
Gastrointestinal tract and rectum Various Enterobacteriaceae except Salmonella, Shigella, Yersinia, Vibrio, and Campylobacter species Glucose nonfermenting gram-negative rods Enterococci α-Hemolytic and nonhemolytic streptococci Diphtheroids S aureus in small numbers Yeasts in small numbers Anaerobes in large numbers (too many species to list) |
| Genitalia Any amount of the following: Corynebacterium species, Lactobacillus species, α-hemolytic and nonhemolytic streptococci, nonpathogenic Neisseria species The following when mixed and not predominant: enterococci, Enterobacteriaceae and other gram-negative rods, S epidermidis, Candida albicans, and other yeasts Anaerobes (too many to list); the following may be important when in pure growth or clearly predominant: Prevotella, Clostridium, and Peptostreptococcus species |
It is likely that microorganisms that can be cultured in the laboratory represent only a fraction of those that are part of the normal resident or transient microbiota. When the broad range polymerase chain reaction (PCR) is used to amplify bacterial 16S rDNA, many previously unidentified bacteria can be detected, as in secretions from patients with bacterial vaginosis. The number of species that make up the normal microbiota has been shown to be much greater than previously recognized. Thus, the understanding of normal microbiota is in transition. As already mentioned, the relationship of previously unidentified microorganisms, which are potentially part of the normal microbiota, to disease is likely to change.
The microorganisms that are constantly present on body surfaces are frequently described as commensals (ie, one partner benefits, while the other seems unaffected). However, in some sites (eg, gut), mutualistic (ie, both parties derive benefit) may be a better description of this relationship. Their flourishing in a given area depends on physiologic factors of temperature, moisture, and the presence of certain nutrients and inhibitory substances. Their presence is not essential to life because “germ-free” animals can be reared in the complete absence of a normal microbiota. Yet the resident flora of certain areas plays a definite role in maintaining health and normal function. Members of the resident microbiota in the intestinal tract synthesize vitamin K and aid in the absorption of nutrients. On mucous membranes and skin, the resident microbiota may prevent colonization by pathogens and possible disease through “bacterial interference.” The mechanism of bacterial interference may involve competition for receptors or binding sites on host cells, competition for nutrients, mutual inhibition by metabolic or toxic products, mutual inhibition by antibiotic materials or bacteriocins, or other mechanisms. Suppression of the normal microbiota clearly creates a partial local void that tends to be filled by organisms from the environment or from other parts of the body. Such organisms behave as opportunists and may become pathogens.
On the other hand, members of the normal microbiota may themselves produce disease under certain circumstances. These organisms are adapted to a noninvasive mode of life defined by the limitations of the environment. If forcefully removed from the restrictions of that environment and introduced into the bloodstream or tissues, these organisms may become pathogenic. For example, streptococci of the viridans group are the most common resident organisms of the upper respiratory tract. If large numbers of them are introduced into the bloodstream (eg, after tooth extraction or oral surgery), they may settle on deformed or prosthetic heart valves and produce infective endocarditis. Small numbers occur transiently in the bloodstream with minor trauma (eg, dental scaling or vigorous brushing). Bacteroides species are the most common resident bacteria of the large intestine and are quite harmless in that location. However, if introduced into the peritoneal cavity or into pelvic tissues along with other bacteria as a result of trauma, they cause suppuration and bacteremia. There are many other examples, but the important point is that the normal resident microbiota is harmless and may be beneficial in their normal location in the host and in the absence of coincident abnormalities. They may produce disease if introduced into foreign locations in large numbers and if predisposing factors are present.
NORMAL MICROBIOTA OF THE SKIN
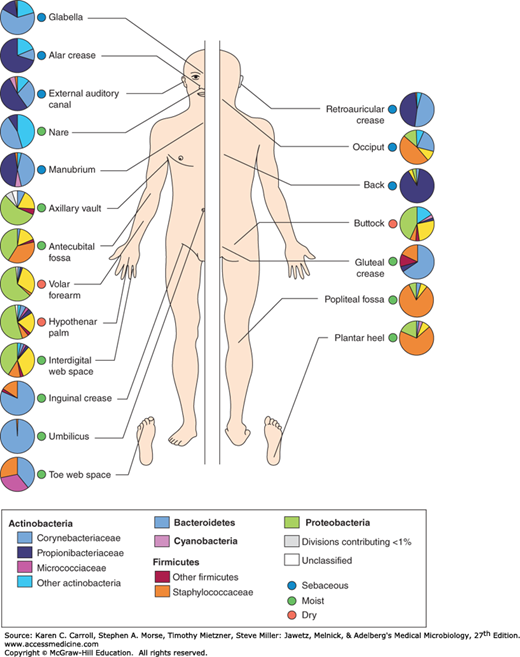
The skin is the human body’s largest organ, colonized by a diverse array of microorganisms, most of which are harmless or even beneficial to the host. Because of its constant exposure to and contact with the environment, the skin is particularly apt to contain transient microorganisms. Nevertheless, there is a constant and well-defined resident flora, modified in different anatomic areas by secretions, habitual wearing of clothing, or proximity to mucous membranes (mouth, nose, and perineal areas) (Figure 10-1).
FIGURE 10-1
Topographical distribution of bacteria on skin sites. The skin microbiome is highly dependent on the microenvironment of the sampled site. The family-level classification of bacteria colonizing an individual subject is shown with the phyla in bold. The sites selected were those that show a predilection for skin bacterial infections and are grouped as sebaceous or oily (blue circles); moist (typically skin creases; green circles); and dry, flat surfaces (red circles). The sebaceous sites are the glabella (between the eyebrows), alar crease (side of the nostril; external auditory canal [inside the ear]), retroauricular crease (behind the ear), occiput (back of the scalp), antecubital fossa (inner elbow), interdigital web space (between the middle and ring fingers), inguinal crease (side of the groin), gluteal crease (topmost part of the fold between the buttocks), popliteal fossa (behind the knee), plantar heel (bottom of the heel of the foot), toe web space, and umbilicus (navel). Dry sites are the volar forearm (inside of the midforearm), hypothenar palm (palm of the hand proximal to the little finger), and buttock. (Reprinted by permission from Macmillan Publishers Ltd: Grice EA, Segre JA, The skin microbiome. Nature Rev Microbiol 2011;9:244-253. Copyright © 2011.)
The predominant resident microorganisms of the skin are aerobic and anaerobic diphtheroid bacilli (eg, Corynebacterium, Propionibacterium); nonhemolytic aerobic and anaerobic staphylococci (Staphylococcus epidermidis and other coagulase-negative staphylococci, occasionally Staphylococcus aureus, and Peptostreptococcus species); gram-positive, aerobic, spore-forming bacilli that are ubiquitous in air, water, and soil; α-hemolytic streptococci (viridans streptococci) and enterococci (Enterococcus species); and gram-negative coliform bacilli and Acinetobacter. Fungi and yeasts are often present in skin folds; acid-fast, nonpathogenic mycobacteria occur in areas rich in sebaceous secretions (genitalia, external ear).
Among the factors that may be important in eliminating nonresident microorganisms from the skin are the low pH, the fatty acids in sebaceous secretions, and the presence of lysozyme. Neither profuse sweating nor washing and bathing can eliminate or significantly modify the normal resident flora. The number of superficial microorganisms may be diminished by vigorous daily scrubbing with soap containing hexachlorophene or other disinfectants, but the flora is rapidly replenished from sebaceous and sweat glands even when contact with other skin areas or with the environment is completely excluded. Placement of an occlusive dressing on the skin tends to result in a large increase in the total microbial population and may also produce qualitative alterations in the flora.
Anaerobes and aerobic bacteria often join to form synergistic infections (gangrene, necrotizing fasciitis, and cellulitis) of skin and soft tissues. The bacteria are frequently part of the normal microbial flora. It is usually difficult to pinpoint one specific organism as being responsible for the progressive lesion because mixtures of organisms are usually involved.
In addition to being a physical barrier, the skin is an immunologic barrier. Keratinocytes continuously sample the microbiota colonizing the skin surface through pattern recognition receptors (eg, Toll-like receptors, mannose receptors, NOD-like receptors). The activation of keratinocyte pattern recognition receptors by pathogen-associated molecular patterns initiates the innate immune response, resulting in the secretion of antimicrobial peptides, cytokines, and chemokines. Despite being constantly exposed to large numbers of microorganisms, the skin can distinguish between harmless commensals and harmful pathogenic microorganisms. The mechanism for this selectivity is unclear.