Source: United States Pharmacopeia and National Formulary (USP34-NF 29). Pharmaceutical compounding—nonsterile preparations. Rockville, Md: United States Pharmacopeial Convention; 2011: 330–6.
Weighing Equipment
By law, your pharmacy must have a prescription balance. This is a scale used to weigh small quantities of solid or very thick semisolid pharmaceutical ingredients (Figure 9-1). Calibration of the scale is the first step in using it—check to make sure the balance has been zeroed, or calibrated, using procedures specific to that scale. Use weighing papers (a type of waxed paper) or cups to keep ingredients from soiling or staining the pans on the scale. Other procedures used on scales vary, depending on the model and type. Ask your pharmacist to give you instructions on how to use the balance in your pharmacy.

Here, you see a prescription balance being used to weigh ingredients for compounding a prescription.
The most common scale present in pharmacies is a Class III balance. If you need to weigh quantities of less than 120 mg, this scale is not sufficiently accurate. Talk with the pharmacist to determine if a different type of scale is available for such small amounts.
Scales must be calibrated periodically. This means that known quantities should be measured on the scale to be sure the scale is accurate. Records should be kept of these calibrations as well as other equipment maintenance.
Measuring Equipment
Your pharmacy should also be stocked with an appropriate supply of equipment for measuring liquids. For most nonsterile products, you will use a graduated cylinder to measure liquids. Several different sizes of these graduates should be available, such as 10 mL, 25 mL, 50 mL, 100 mL, and 1000 mL (Figure 9-2). To ensure greater measurement accuracy, you should select a graduate that has a 20% greater capacity than the amount you are measuring. For instance, you would not measure 3 mL in a 100 mL graduate; rather you should use a 10 mL graduate. For measuring amounts of 0.1 mL to 5 mL, you may use syringes (without a needle attached). For measuring very small amounts of liquids (less than 0.1 mL), use micropipettes.

The two taller containers shown in this photograph should be used for measuring volumes of liquids in the pharmacy. The pharmaceutical graduate, shown on the right, can be used to measure liquids when accuracy is not as important, such as when an antibiotic suspension is being reconstituted. The beaker, shown in the front, should be used only for holding liquids because it is not sufficiently accurate for measuring liquids.
Compounding Equipment
The pharmacy must also have an adequate supply of mortars and pestles (with both pieces made of glass or Wedgwood/porcelain), stainless steel and plastic spatulas of different sizes, an ointment slab or pill tile, funnels, filter paper, beakers, glass stirring rods, a heat source (hot plate or microwave oven), a refrigerator, and a freezer. These are used in various steps of the compounding process, as directed. Some of these compounding elements are shown in Figure 9-3.
Ingredients
The pharmacist will generally stock the compounding area with chemicals that meet requirements of USP, National Formulary (NF), or Food Chemicals Codex (FCC) substances.1 Ideally, these ingredients should be manufactured in a facility registered with Food and Drug Administration (FDA). USP, NF, and FCC list the standards the chemical must meet for strength, quality, and purity. FDA inspects the plants in which the chemicals were made for compliance with its “Good Manufacturing Practice” standards, ensuring that the chemicals are suitable for use in people. When selecting products for compounding, be sure that you pick the correct salt (for example, sodium chloride versus sodium phosphate) called for in the prescription. If you are crushing commercially available tablets to obtain a needed drug, do not use extended-release or delayed-release products.
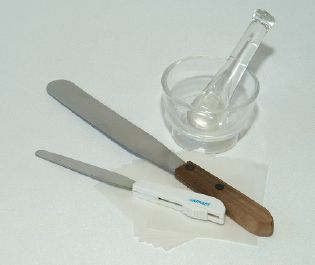
Spatulas and a mortar and pestle, like the glass one shown here, are used in compounding. A mortar and pestle can also be made of porcelain; in this case it is called a Wedgwood mortar and pestle.
Techniques Used in Nonsterile Compounding
Many pharmaceutical ingredients can be mixed without any problems of compatibility. However, certain ingredients must be mixed carefully or in a special way to prevent problems in compounding. Pharmacists spend a considerable amount of time in pharmacy school learning the principles and techniques used in these situations. While these principles and techniques cannot be detailed in this text, the following are brief descriptions of the basic techniques used most commonly to mix pharmaceutical ingredients.
Trituration
Trituration is a grinding of a drug solid using a mortar and pestle to reduce the particle size or to mix two or more solids (Figure 9-4). This technique is generally used to
- Create a finer powder to make dissolution easier;
- Keep a cream or ointment from feeling gritty; or
- Ensure thorough mixing of solid ingredients.
Levigation
Levigation entails dispersing a drug solid into a small amount of mineral oil, glycerin, or other liquid before incorporating the paste into an ointment (often by trituration). This technique is used to keep the ointment from feeling gritty.
Geometric Dilution
Geometric dilution is a technique used to ensure even mixing of a small amount of a potent drug with a large amount of a nonpotent or inactive compound. The potent drug is triturated with an approximately equal amount of the other substance. That mixture is then combined with an approximately equal amount of the other substance and so forth, until all of the second substance is incorporated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


