Compound
Usual dose
Onsetof action (min)
Durationof action
Activation
Glycerol Trinitrate (GTN)
Sublingual0.3–0.6 mg
2–5
10–30 min
Mithocondrial activation
Spray 0.4 mg
1–2 sprays as needed
2–5
10–30 min
Transdermal patch 0.2–0.4 mg/h
30
8–14 h
Isosorbide Dinitrate (ISDN)
Oral 5–8 mg
Twice to thrice daily
15–30
3–6 h
Cytochrome p450 biotransformation
Isosorbide Mononitrate (ISMN)
Oral 20 mg
Twice daily(7 h apart)
30–60
12–14 h
Cytochrome p450 biotransformation
Pentaerythrityl tetranitrate (PETN)
Oral 50–80 mg
Twice to thrice daily
20–30
10–12 h
Mithocondrial activation
Mechanisms of Action
Nitrates dilate veins, arteries, and coronary arteries by relaxing vascular smooth muscle [4]. Among the currently available compounds, the organic nitrates glyceryl trinitrate (GTN) and pentaerythrityl tetranitrate (PETN) are pro-drugs metabolized by the mitochondrial and cytosolic aldehyde dehydrogenase (ALDH-2); ALDH-2 acts as a GTN-reductase that calalyzes the bioactivation of GTN into 1,2-GTN through a NO-independent pathway (Table 5.1). Isosorbide dinitrate (ISDN) and isosorbide mononitrate (ISMN) undergo denitrification through an as yet unknown mechanism, which is not affected by ALDH-2 inhibition (or genetic deletion) [5, 6] ISDN major metabolites, isosorbide-2-mononitrate and isosorbide-5-mononitrate, are both biologically active, with half-lives of approximately 2 and 4 h, respectively. ISMN does not undergo first-pass hepatic metabolism and is completely bioavailable. The vasodilation which follows the administration of organic nitrates is mediated by the activation of soluble guanylate cyclase, followed by increased formation of cyclic guanosine-3′,-5′-monophosphate (cGMP) and activation of cGMP-dependent protein kinases and/or cyclic nucleotide- gated ion channels [7, 8] (Fig. 5.1)
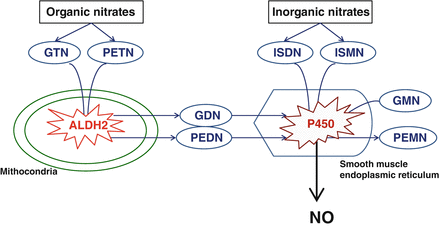

Figure 5.1
Organic (high potency) nitrates (GTN, PETN) are bioactivated by mitochondrial and cytosolyc ALDH-2. The reductase activity converts the organic nitrates to the denitrated metabolites (GDN, PEDN). The low-potency nitrates (ISDN, ISMN, GDN, PEDN, and their respective mononitrates (GMN and PEMN) are bioactivated by P450 enzymes in the endoplasmic reticulum directly yielding NO. The latter mechanism also accounts for the high potency nitrates used at high doses. GTN glyceryl trinitrate, PETN pentaerythrityl tetranitrate, ALDH aldehyde dehydrogenase, GDN glyceryl dinitrate, PEDN pentaerythrityl dinitrate, ISDN isosorbide dinitrate, ISMN isosorbide mononitrate, GMN glyceryl mononitrate, PEMN pentaerythrityl mononitrate, NO nitric oxide
Effect on Systemic Hemodynamics
Organic nitrates exert their maximal vasodilatory effect at the level of venous capacitance vessels, large and medium-sized coronary arteries and collateral vessels, while arterioles with a diameter <100 mm are relatively less affected (Table 5.2). Venodilation lowers preload (left ventricular end-diastolic pressure) and therefore reduces wall stress, resulting in a decrease in myocardial oxygen demand, which is of benefit in subjects affected by effort induced angina caused by severe coronary artery disease. The fall in preload is more pronounced with sitting or standing and this may lead to postural hypotension.
Table 5.2
Nitrates: mechanism of action
Hemodynamic actions | Non-hemodynamic actions | |||
|---|---|---|---|---|
Preload reduction | Improvement of myocardial oxygen supply | Afterload reduction | Preconditioning-like effect | Platelet inhibition |
Venous return (−) | Dilation of collaterals | Systolic pressure (−) | ||
LV and RV EDP (−) | Dilation of eccentric coronary stenosis | Systolic stress (−) | ||
Diastolic wall stress (−) | Aortic compliance (+) | |||
At low doses, nitrates cause a lesser degree of arterial and arteriolar dilation, leading to little or no change in systemic vascular resistance or blood pressure [4]. As the dose is increased, the blood pressure falls, often accompanied by reflex tachycardia. Wall stress is reduced at the lower blood pressure, resulting in a further decrease in myocardial oxygen demand.
Effect on Coronary Hemodynamics
A nitrate-induced increase in coronary blood flow has been proposed as a potential mechanism for relieving ischemia. Animal and human studies have shown that nitrates dilate both normal and abnormal coronary arteries [9]; this response is preserved in saphenous vein grafts [10]. The effect of nitrates on the arteriolar system is uncertain because the coronary arterioles in patients with severe flow-limiting coronary stenoses are already maximally dilated in an attempt to maintain resting blood flow at an appropriate level. There are, however, settings in which a direct effect of nitrates on coronary hemodynamics is clearly beneficial. For example, in the acute coronary syndromes, the dilation of coronary collaterals and conductance arteries can increase oxygen supply [11]. Similar hemodynamic effects provide a background for the benefit of organic nitrates in patients with congestive heart failure. In this setting, the redistribution of blood from the central circulation into large capacitance veins decreases right atrial pressure, and the dilation of large conduit arteries improves the impedance to the left ventricular ejection. The ensuing reduction in left ventricular end-diastolic pressure and left ventricle wall tension concurs to the hemodynamic stabilization of decompensated heart failure [12].
Nitrates can also reduce or reverse coronary artery vasospasm [13]. Thus, patients with primarily vasospastic angina or a large vasoconstrictor component to their angina can benefit from the direct coronary action of nitrate therapy.
Non-hemodynamic, Anti-ischemic Effects of Organic Nitrates: Preconditioning-Like Phenomena and Platelet Inhibition
The term ‘ischemic preconditioning’ describes a protective phenotype that is characterized by a reduced sensitivity to ischemia and reperfusion injury. While this protective phenomenon is, in its traditionally accepted form, triggered by the exposure to a short period of ischemia (such as angina), some drugs have also been shown to possess similar effects, suggesting that the pharmacological manipulation of the ischemic threshold at a cellular and whole organ level could be used as a tool in the prevention or reduction of ischemic events (Table 5.2). Several lines of evidence from both animal and human studies have now clearly demonstrated that the administration of nitroglycerin is associated, independently of any vasodilator effects, with an increased ischemic threshold, as manifested by reduced infarct size, reduced ECG changes in the setting of percutaneous angioplasty, and reduced endothelial dysfunction after ischemia and reperfusion [14–18]. These observations might have direct clinical implications: for instance in the setting of angioplasty or coronary artery bypass grafting, but possibly also during chronic therapy, as shown in a recent post hoc analysis of the GRACE trial and in another smaller retrospective study [19, 20].
Besides these effects, nitrates also reduce platelet aggregability ex vivo in healthy volunteers and, to a lesser extent, in patients with coronary artery disease, which might be particularly useful in the setting of acute coronary syndromes. Whether the antiaggregant effects of nitrates have an additional clinical impact in the therapy of coronary artery disease, particularly in light of the introduction and systematic use of targeted antiplatelet agents such as aspirin, and thienopiridines is, however, unclear.
Nitrate Tolerance
The clinical use of nitrates is limited by the development of tolerance, i.e., the loss of hemodynamic and symptomatic effects that invariably occurs upon prolonged (>12 h) treatment. In the setting of coronary artery disease, this also translates in the loss of antianginal effects. Another phenomenon, this time caused by the withdrawal of chronic nitrate therapy i.e. ‘rebound’ effect [21] results in the worsening of the patient’s anginal symptoms.
Mechanisms of Tolerance
The mechanism responsible for nitrate tolerance is incompletely understood. Tolerance appears to be a complex phenomenon involving vascular, biochemical, and autonomic changes with oxidative stress playing a central role [22, 23], as demonstrated by the fact that administration of vitamin C prevents or reverses these modifications [24, 25]. Tolerance is due to attenuation of the vascular effect of nitrates, but not to altered pharmacokinetics, with at least three (not mutually exclusive) proposed mechanisms [1]:
1.
Impaired nitroglycerin bioconversion to 1,2-glyceryl dinitrate with decreased formation of NO. This effect is nitrate-specific and is not seen with non-nitrate sources of NO such as nitroprusside [26]. Consistent with this theory are the experimental observations that there is no tolerance to the effect of S-nitrosothiols and that the activity of mitochondrial aldehyde dehydrogenase-2 (mtALDH), the enzyme required for metabolism of nitrates to 1,2 glyceryl dinitrate in markedly reduced [5]. The same findings can be induced by inhibitors of ALDH [5].
2.
Reduced bioactivity of NO [27]. This is supported by the finding that vascular and hemodynamic tolerance to nitrates occurs in animals despite high levels of NO and rates of NO formation that were similar in those animals that were not tolerant [28]. In addition, transgenic animals that overexpress endothelial NO synthase have chronically elevated NO release, which is associated with reduced vascular reactivity to NO-mediated vasodilators [29].
3.
Activation of the renin-angiotensin-aldosterone system and sympathetic nervous system in response to nitrate-induced vasodilation [30, 31]. The increased peripheral sensitivity to these vasoconstrictors can be reversed by angiotensin converting enzyme inhibition [30].
Abnormal coronary vasoconstrictor responses have also been described with continuous nitrate exposure [32].
Prevention
Although the mechanisms of nitrate tolerance remain unknown, several approaches to its prevention have been studied. The only widely accepted and most effective method of preventing tolerance is the use of a dosing strategy that provides an interval of low nitrate exposure during each 24-h period. It is thought that a nitrate–free interval permits the regeneration of reduced sulfhydryl groups, thereby restoring vascular responsiveness to nitrates (Table 5.3).
Table 5.3
Nitrates: side effects and precautions of therapy
Side effect | Comment | Contraindications |
|---|---|---|
Headache | It often responds after several days of therapy | |
Resolution of headache does not necessarily mean loss of efficacy | ||
Postural hypotension | Initiate treatment with small doses and increase as necessary | Coadministration of phosphodiesterase-5 inhibitors |
Light-headedness | Dose reduction may be required | Hypertrophic cardiomyopathy |
Syncope | Caution in the elderly, severe aortic stenosis or volume depletion | Right ventricular infarction |
Flushing | Initiate treatment with small doses and increase as necessary | Allergic reactions to organic nitrates |
Local redness Mild inflammation | Vary application site | Allergic reactions to organic nitrates |
Tolerance | Several strategies have been proposed to avoid tolerance: | |
Nitrate-free interval | ||
N-acetylcisteine | ||
Folic acid | ||
l-arginine | ||
Hydralazine | ||
Antioxidants: vitamin E, vitamin C, carvedilol | ||
Statins | ||
Angiotensin converting enzyme inhibitors | ||
Diuretics | ||
Nitrate rebound | These occur during nitrate-free period. Research Some lines of research provide a potential role of PETN in these cases. | Caution in the early days of acute coronary syndrome |
Time zero effect |
There are, however, two concerns regarding intermittent therapy:
1.
A time-zero effect, which refers to a deterioration in exercise performance relative to placebo prior to the morning dose of nitrates. In a study of 215 patients given nitroglycerin or placebo patches, the time spent walking on the treadmill in the morning increased before application of the patch in the placebo group but not in the nitroglycerin group, suggesting that withdrawal of nitroglycerin had an adverse effect on exercise performance [33]. This effect was confirmed in other studies of transdermal nitroglycerin therapy [21, 34]. Adverse effects on exercise tolerance have not been reported in studies of other long-acting nitrates given once daily or in eccentric dosing regimens [9, 35].
Whether these effects occur to a clinically significant degree remains unclear and no firm conclusions can be drawn concerning the risk of acute ischemic events during intermittent nitrate therapy [21, 38–40]. Despite this uncertainty, patients and their physicians should be aware of the fact that the nitrate-free period during intermittent dosing regimens should be associated with increased angina.
Other pharmacologic interventions have been tested to reduce nitrate tolerance, although none is as yet used clinically:
Chronic therapy with N–acetylcysteine, a sulfhydryl donor, does not appear to be effective in patients with stable angina [41], in contrast to its acute benefit with intravenous nitroglycerin in subjects with unstable angina.
Folic acid can reverse endothelial dysfunction, possibly by restoring the bioavailability of tetrahydrobiopterin, a cofactor for NO synthase and/or arginine, its substrate. This suggests a possible role for folic acid in preventing nitrate tolerance. This was examined in a study of 18 subjects who were randomly assigned to folic acid (10 mg/day) or placebo for 1 week; all patients received continuous transdermal nitroglycerin (0.6 mg/h) [42]. Compared to placebo, folic acid prevented the development of both endothelial dysfunction and nitrate tolerance.
Treatment for 5–10 days with l–arginine, the substrate for NO synthesis, can modify or prevent the development of nitrate tolerance during continuous transdermal nitroglycerin use [43].
Hydralazine may attenuate nitrate tolerance, perhaps by preventing superoxide generation [44]. This relationship could contribute to the efficacy of combined nitrate-hydralazine therapy in patients with heart failure. In patients with angina pectoris, hydralazine should be given in combination with a beta blocker because of the reflex sympathetic activation.
Other antioxidants may be helpful, at least from a theoretical perspective, such as vitamin E [ 45] and vitamin C [ 46, 47]. In addition, carvedilol, a beta and alpha blocker that also has antioxidant activity, may prevent nitrate tolerance [48]. The importance of antioxidant activity was suggested by a second report which compared carvedilol with another beta and alpha blocker (arotinolol) that was devoid of antioxidant properties; only carvedilol prevented nitrate tolerance [49].
Therapy with statins has been associated with a number of benefits that are independent of their effect on lipid levels. In animal studies, both pravastatin and atorvastatin prevented nitrate tolerance and vascular superoxide formation induced by subcutaneous GTN injections [50] an effect that was associated with increased basal cGMP levels and was abolished when the rats received an inhibitor of the eNOS concomitantly with GTN. Therapy with statins also appears to improve platelet reactivity to GTN in patients with stable or unstable coronary syndromes [51].
Commonly Used Nitrate Preparations
Numerous nitrate preparations are commercially available, including sublingual, buccal, oral, spray, ointment, and transdermal preparations (Table 5.1).
Glyceril Trinitrate
Sublingual Administration
Sublingual nitroglycerin dilates capacitance veins and conduit arteries, relieving symptoms of angina and decreasing cardiac oxygen demand. These effects are so consistent and reproducible that a symptomatic improvement immediately after the administration of sublingual nitroglycerin or isosorbide dinitrate is considered to represent valuable information in the differential diagnosis of chest pain [56]. Similarly, the use of sublingual nitrates in settings where angina is anticipated (i.e. in the symptomatic prophylaxis) is also strongly recommended, and physicians should educate and encourage patients to self-administer nitrates before performing physical activity, or in cases of emotional stress.
The onset of action is within 2–5 min and the duration of action is 10–30 min. Tolerance is not a problem with sublingual nitroglycerin because of its intermittent administration, even in patients on chronic nitrate therapy [57].
The recommended nitroglycerin dose is 0.3 mg (1/200 grains) to 0.6 mg (1/100 grains). One half the dose (0.15 mg or 1/400 grains) can be used if the patient becomes hypotensive or develops symptoms such as headache or flushing with the higher doses. Elderly patients should be warned about potential lightheadedness, especially in warm weather.
The traditional recommendation is for patients to take one nitroglycerin dose sublingually every 5 min for up to three doses before calling for emergency medical services (EMS) evaluation. However, studies suggest that this approach may result in significant delays in obtaining EMS assistance [58, 59]. As a result, the 2004 College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend contacting EMS if chest pain or discomfort is unimproved or worsening 5 min after one nitroglycerin dose has been taken [60]. No change to this approach was made in the 2007 focused update of the 2004 ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction [61]. For patients known to their providers to have frequent angina, physicians may consider a selected, more tailored message that takes into account the frequency and character of the patient’s angina and their typical time course of response to nitroglycerin.
If the sublingual nitroglycerin is potent, a slight tingling sensation should be felt under the tongue. Tablets that crumble easily should not be used. The sublingual mucosa should be moist for adequate dissolution and absorption of the tablet. A drink of water in patients with dry sublingual mucosa prior to ingestion of the tablet may be necessary.
Nitroglycerin tablets are both heat and light sensitive. They should therefore be stored in a tightly capped dark bottle in the refrigerator with only a small supply being carried by the patient. Nitroglycerin tablets in an opened bottle should be discarded after 12 months.
Patient education is extremely important for the proper use of sublingual nitroglycerin. A survey of 50 patients revealed a surprising lack of knowledge concerning the administration, storage, and side effects of this preparation [62]. Only 12 % knew the maximum dose in a 15 min period, 28 % knew the proper storage conditions for sublingual tablets, and 52 % knew the most common side effects.
Sublingual Spray
An equally effective means of administering sublingual nitroglycerin is by metered dose spray. The spray dispenses of 0.4 mg of nitroglycerin. One to two sprays can be used at the start of an attack and up to three sprays can be used in a 15 min period. It has a shelf life of 2–3 years [63].
Transdermal Patch
Transdermal nitroglycerin patches obtained US Food and Drug Administration (FDA) approval in 1981 and gained wide acceptance for its convenience. These patches have either a polymer matrix or a silicone gel impregnated with nitroglycerin; a semipermeable membrane between the drug reservoir and the skin results in a constant delivery of nitroglycerin. Onset of action is 30 min with duration of action in the region of 8–14 h. The usual dose is 0.2–0.8 mg/h [64]. Once again, continuous therapy is associated with nitrate tolerance. In one report, for example, patches of 5, 10, 15, 30, and 45 mg were given continuously and treadmill walking time was examined after 2, 4, and 24 h. With the exception of the 45 mg dose, antianginal effects were seen at 2 and 4 h but were absent at 24 h on the first day. Daily therapy with 15 mg patches for 1–2 weeks was associated with antianginal and hemodynamic effects that were not different from placebo patches. Similar findings were noted in a multicenter, randomized, placebo controlled trial involving 562 patients examined the efficacy of 8 weeks of continuous transdermal nitroglycerin [65]. In doses ranging from 15 to 105 mg/day, there was no difference in exercise tolerance or anginal frequency between active drug and placebo; this effect was noted within 24 h. As a result of these findings, intermittent transdermal regimens have been evaluated. A recent trial evaluated the efficacy of three dose levels of transdermal nitroglycerin (0.2, 0.4, and 0.8 mg/h) applied for 12 h daily for 30 days in 291 patients with chronic stable angina. After 30 days of therapy, treadmill-walking time until the onset of angina or 1 mm ST depression was significantly improved in all treatment groups when compared to placebo. In addition, there was no evidence of rebound angina or partial tolerance when the antianginal effects on day 30 were compared to those on day [21]. This trial did not directly compare patch-on and patch-off periods and hence the problem of rebound angina after transdermal nitroglycerin withdrawal was not adequately addressed. However, another study, directly evaluated the effect of intermittent transdermal nitroglycerin on the occurrence of ischemia during patch-off hours in 72 patients who were randomized to 12 h of transdermal nitroglycerin or placebo [66]. After 2 weeks of therapy, patients crossed over to the alternative treatment. Compared to placebo, transdermal nitroglycerin significantly reduced the magnitude of ST segment depression at angina onset during exercise testing, but did not alter total angina frequency. Angina frequency and silent ischemia increased by 14 % during patch-off hours compared to patch-on hours.
Isosorbide Dinitrate
Isosorbide dinitrate (ISDN) has an onset of action within 15–30 min and the duration of action is 3–6 h. Low bioavailability from hepatic metabolism has necessitated relatively large doses of 10–40 mg three times daily. The beneficial effects of a single dose of ISDN (15, 30, 60, 120 mg) were demonstrated in 12 patients with chronic stable angina [57]. There was a dose-related reduction in systolic blood pressure that persisted for 8 h. Exercise duration improved up to 8 h after the 15 and 30 mg dose; there was no added benefit with the 60 and 120 mg doses. Unfortunately, tolerance has limited the usefulness of ISDN as a chronic antianginal agent. In the study above, ISDN was given four times daily for 2 weeks [67]: both the blood pressure and exercise responses were attenuated. In particular, exercise duration was only increased for 2 h after a dose and doses above 15 mg four times daily produced no added benefit. The development of tolerance occurred despite higher plasma concentrations of ISDN during maintenance therapy. Several studies have altered the drug regimen in an attempt to prevent the development of tolerance. One study, for example, examined the effect of 30 mg of ISDN given two (7 am and 12 pm), three (7 am, 12 pm, and 5 pm), and four (7 am, 12 pm, 5 pm, and 11 pm) times daily for 1 week [68]. Exercise duration until the onset of angina was assessed before and 1, 3, and 5 h after the morning dose. After a single initial dose, exercise duration significantly increased versus placebo over the 5 h observation period. After 1 week of therapy, two and three (but not four) times daily dosing was associated with improved exercise tolerance compared placebo; however, the benefit was less pronounced late in the day, indicating partial tolerance. One limitation to the clinical utility of these results is that the response was measured only to the morning dose of ISDN.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


