Figure 6.1
Chemical structure of nicorandil
Mechanism of Action
Anti-Angina Actions
Nicorandil has two distinct anti-angina mechanisms (Fig. 6.2) [1–3]. The nitrate component acts mainly on systemic venous (capacitance) vessels to reduce preload, and also dilates epicardial coronary arteries. In this context, vasodilatation occurs due to activation of soluble guanylate cyclase, resulting in increased cGMP, activation of protein kinase G, and subsequent lowering of intracellular Ca2+ and inhibition of myosin light-chain kinase activity; ultimately leading to relaxation of vascular smooth muscle cells [4]. The nitrate-like effect of nicorandil on the cGMP pathway accounts for the majority of its anti-angina effects at therapeutic concentrations [5].
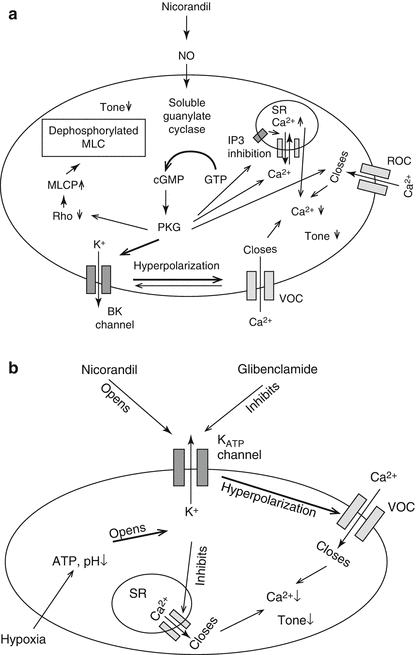

Figure 6.2
Molecular actions of nicorandil, showing activation of both cGMP Cyclic GMP (guanosine monophosphate) (a) and K+ ATP (b) signaling pathways. MLC myosin light chain, ROC receptor-operated channels, VOC voltage-operated channels, BK big potassium, ATP adenosine triphosphate, IP3 inositol triphosphate, SR sarcoplasmic reticulum, GTP guanosine-5’-triphosphate, PKG protein kinase G (Figure adapted from Horinaka [20])
Nicorandil is also a K+ ATP channel opener. K+ ATP channels control cell excitability, and by opening these channels, nicorandil causes vascular smooth cell depolarization and indirect closure of L-type voltage gated calcium channels resulting in arterial dilatation (similarly to calcium channel blockers) [6, 7]. This affects peripheral and coronary arterial resistance vessels (primarily the coronary microcirculation), resulting in decreased afterload and improved coronary flow [8–10].
Unlike nitrates, tolerance does not develop with nicorandil, probably due to its dual mode of action [11]. This is of particular importance in view of the long-term deleterious effects on endothelial function reported due to free radical accumulation in the context of nitrate tolerance [12]. Sekiya et al. studied the long-term effects of nicorandil and isosorbide dinitrate on endothelial function in 42 patients with ischaemic heart disease in 2005 [13]. In this study, isosorbide dinitrate was associated with significantly worse flow mediated dilatation (a marker of endothelial function) and carotid intima-medial thickness at 3 months, whereas nicorandil showed improved endothelial function and no progression of atherosclerosis.
Cardio-Protective Mechanisms
The actions of nicorandil on K+ ATP channels confer cardio-protection through activation of pathways linked to ischaemic preconditioning, however the exact mechanism for this remains unclear [14, 15]. During periods of myocardial ischaemia, KATP channels are opened due to decreased in ATP, cellular acidosis and actions of adenosine mediated via A1 receptors. Hypoxic dilatation of the coronary arteries is mediated by KATP channels [16]. Activation of KATP channels also shortens myocardial cell action potentials (seen as electrocardiographic shift in ST-segments), reduces Ca2+ overload and cellular energy demands; this has been proposed as an endogenous anti-ischaemic mechanism that may, in part, explain the cardio-protective properties of nicorandil [17]. However, more recently, attention has focused on the role of mitochondrial KATP channels in mediating ischemic preconditioning due to nicorandil [18, 19], and in particular its potential to reduce oxidative stress through inhibition of mitochondrial permeability transition pore (mPTP) activation during ischaemic reperfusion injury [20, 21].
Haemodynamic Effects
The haemodynamic effects of nicorandil have been studied in patients undergoing coronary angiography, which overall result in balanced offloading of the ventricles through reduction in preload and afterload, and improved coronary flow due to lowered coronary arterial resistance. A mild transient, dose-dependant sympathetically medical baroceptor reflex tachycardia can also occur, but nicorandil does not directly affect cardiac conduction or contractility [22].
Nicorandil dilates coronary arteries on average by 10–20 % in patients with coronary artery disease, which is mostly due to its nitrate-like effect [23]. Significant reduction in coronary arterial resistance is also observed [24, 25]. Coltart et al. showed in a study of 15 patients undergoing routine angiography in 1989, that administration of 40 mg of nicorandil decreased preload (left ventricular end diastolic pressure lowered 4.8 mmHg and mean pulmonary artery pressure 5.7 mmHg), afterload (total peripheral resistance lowered 19 %) and blood pressure (systolic pressure lowered 34 % and diastolic 21 %) [26]. In other angiographic studies, 20 mg of nicorandil decreased left ventricular systolic pressure by 12–13 %, and increased non-stenotic and stenotic epicardial artery diameter by 14 % [27]. Nicorandil has also been shown to significantly improve cardiac index in patients with congestive heart failure [28], and regional wall motion abnormalities following myocardial stunning [29].
Pharmacokinetics
Nicorandil is rapidly and almost completely absorbed via the gastrointestinal tract, reaching maximal plasma concentration after 30–60 min, and stead-state levels following 4–5 days of standard therapy. Gastrointestinal absorption is delayed, but not decreased by food. Its half-life is 52 ± 13 min. Nicorandil does not undergo first-pass metabolism, and displays a linear dose-to-plasma concentration relationship at doses of 5–40 mg. The oral bioavailability is 75 ± 23.6 %. Metabolism is mainly via denitration into the nicotinamide pathway, with less than 20 % of the administered dose excreted in the urine. Nicorandil circulates largely unbound to albumin or other plasma proteins. Its anti-angina effects last approximately 12 h, necessitating twice-daily dosage. Pharmacokinetic properties are unchanged in the elderly, and in chronic liver and/or renal disease. Plasma levels are not significantly altered by any known drug interactions [30, 31].
Practical Considerations
Dosage
The recommended starting dose of nicorandil is 10 mg twice daily (Table 6.1). In particular patients susceptible to headache, a starting dose of 5 mg twice daily may be used, with subsequent up-titration to achieve clinical response. The usual therapeutic dose is 10–20 mg twice daily, although up to 30 mg twice daily may be used if necessary. As a general principle the lowest effective dose is recommended to avoid potential side-effects, especially in the elderly; although no specific dose adjustments appear to be required for age, or with hepatic or renal impairment.
Table 6.1
Nicorandil prescribing
Indications |
To prevent symptoms of stable angina (second-line drug) |
To prevent cardiac events in patients with stable angina and atherosclerosis |
Dose |
Starting dose: 10 mg twice daily |
Maintenance dose: 10 to 20 mg twice daily |
No dose adjustment required for hepatic or renal impairment |
Side–effects |
Common: headache, dizziness, flushing, malaise, nausea/vomiting |
Rare: gastro-intestinal tract, skin and mucosal ulcers |
Contraindications |
Hypotension/cardiogenic shock |
Aortic stenosis |
Not to be co-administered with phosphodiesterase-5-inhibitors (e.g. sildenafil); may cause profound hypotension |
Caution advised when combining with other drugs that potentially lower blood pressure |
Safety and Tolerability
The safety of nicorandil, as monotherapy and when combined with other anti-angina agents, has been demonstrated by numerous clinical trials, including a large Prescription Event Monitoring (PEM) study [32]. There is a theoretical risk of arrhythmia due to shortening of the myocardial action potential, however no adverse effect on rhythm has been observed in clinical studies and nicorandil may, in fact, protect against ischaemic-induced arrhythmias in patients with unstable coronary disease [33].
Nicorandil is reasonably well-tolerated by most patients; less than 10 % of patients report side-effects at 30 days, and on average 70 % continue to take the medication at 1 year [34]. The side-effect profile is mostly related to its action as a vasodilator. Headache is the most common side-effect, and main reason patients stop taking nicorandil. Headaches are reported by 36 % of patients, usually within the first few days of therapy, and can often be prevented when using a lower starting dose with slow up-titration. Indeed, symptomatic lowering of blood pressure tends only to occur with starting doses ≥40 mg. Other common side-effects are dizziness, flushing, malaise and gastro-intestinal upset, which occur in ≤3 %. Rarely, gastrointestinal, skin and mucosal ulcers may develop [35, 36]; these may be refractory to treatment and respond only to withdrawal of nicorandil, which should be stopped in this context. Overall, the incidence of side-effects is similar to other anti-angina agents, including beta-blockers, calcium channel blockers and long-acting nitrates. Nicorandil does not cause worsening of angina, and there is no rebound phenomenon when discontinued abruptly [37].
Cautions and Contraindications
Nicorandil is contraindicated in patients who are allergic to the drug or its constituents. It must not be used in cardiogenic shock, hypotension, or left ventricular failure with low filling pressure, and is also contraindicated by aortic stenosis. Due to the risk of profound hypotension, nicorandil should not be used in conjunction with phosphodiesterase-5-inhibitors (e.g. sildenafil), and caution should be taken when co-administered with other agents that potentially lower blood pressure. As there is a small, but potential risk of gastro-intestinal ulceration due to nicorandil, caution is advised when prescribing for patients also taking corticosteroids. There are no other specific drug warnings, however it is important to note that sulphonylureas have the potential to close K+ ATP channels and thus may antagonize the effects of nicorandil.
The effects of nicorandil during pregnancy, breast-feeding and on fertility have not been studied in humans; although no teratogenicity or negative effects on fertility have been observed in animal studies. Therefore, the risks versus benefits of prescribing during pregnancy must be carefully considered. Nicorandil is not recommended while breast-feeding, as it is not known whether it is excreted into human milk.
Clinical Indications and Guidelines
Nicorandil is available in many European countries and the United Kingdom, but is not yet licensed in the United States. Clinical indications are: (1) prevention and long-term treatment of chronic stable angina, and (2) reduction in risk of acute coronary syndrome in patients with stable angina and (a) previous myocardial infarction, (b) coronary artery by-pass grafting surgery, and/ or (c) angiographic evidence of coronary artery disease, or (d) positive exercise test together with cardiovascular risk factors.
ESC (European Society of Cardiology) guidance lists a class I (is recommended) indication for nicorandil for patients with intolerance, or contra-indication to beta-blockers and calcium channel blockers (level of evidence C), and class IIa (should be considered) for those with symptoms despite treatment with first agents (level of evidence B) [38]. NICE (National Institute for Health and Clinical Excellence), similarly, recommend the use of nicorandil for these purposes [39].
Clinical Efficacy
Nicorandil is effective in treating the symptoms of stable angina; both typical, effort-induced symptoms originating from obstructive epicardial artery atherosclerosis (and/or microvascular disease), and angina at rest due to coronary spasm. Its cardio-protective properties offer additional prognostic benefit, to help prevent cardiac events in patients with stable angina; and possibly to protect against ischemic-reperfusion injury and arrhythmia in those with unstable coronary disease.
Effort-Induced Angina
The clinical efficacy of nicorandil to improve symptoms of effort–induced angina has been evaluated by a number of clinical trials over the past several decades, which overall have shown similar results to conventional anti-angina drugs, with equal (or potentially less) side-effects and without development of tolerance. The overall efficacy in effort-induced angina is reportedly 71 % [40, 41].
Initial non-comparative studies [42, 43], and prospective randomized, double-blinded studies comparing nicorandil to placebo showed significant improvement on exercise testing from baseline in patients with stable angina [44–48]. In the largest of the placebo-controlled studies, which included 46 patients with stable angina, Meany et al. observed significantly increased time-to-angina in the study group following 2 weeks of nicorandil at 2 h (38 % vs. 2 %, p < 0.05) and 12 h (23 % vs. 2 %, p < 0.05) after administration (Fig. 6.3). In comparison to long-acting nitrates (isosorbide mononitrate and isosorbide dinitrate) [49–51], calcium channel blockers (nifidipine, amlodipine and diltiazem) [52–54], and beta-blockers (atenolol, propranolol and metoprolol) [55–57], nicorandil has shown consistently similar short-term anti-ischaemic efficacy.
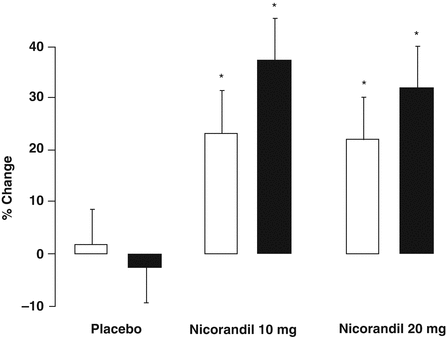

Figure 6.3
Effect of nicorandil after 2 weeks of twice-daily therapy, showing significantly increased time-to-onset of angina during exercise at baseline (light bars) and 2-h post dose (black bars). *p<0.05 (Figure adapted from Meany et al. [47])
Among the larger trials to evaluate nicorandil, were the SNAPE (Study of Nicorandil in Angina Pectoris in the Elderly) and SWAN (Comparison of the anti-ischaemic and anti-anginal effects of nicorandil and amlodipine in patients with symptomatic stable angina pectoris) studies, carried out in 2000 and 1999 respectively [50, 53]. The SNAPE study, which included 194 patients with stable angina, reported a similar increase in time-to-ischaemia and time-to-angina, and decrease in maximum ST-segment depression on symptom-limited bicycle exercise test after 4 weeks of treatment with nicorandil versus isosorbide mononitrate [50]. The SWAN study was a multi-centre, double-blind, randomized study comparing nicorandil and amlodipine in 121 patients with stable angina from 25 centres in Austria and Switzerland over 8 weeks [53]. In this study, time-to-symptoms and total exercise duration was again similarly increased among the two study groups. Nicorandil also resulted in improved quality of life variables (similar to amlodipine), and a marginally more favourable tolerability profile.
For patients with effort-induced angina and evidence of inducible myocardial ischaemia, despite angiographically unobstructed coronary arteries (i.e. Cardiac syndrome X), nicorandil may also be used. In these patients, nicorandil augments coronary flow reserve by dilating the coronary microvasculature, ameliorating exercise-induced ischemia and related symptoms [58]. Nicorandil may also help to prevent against iatrogenic microvascular damage in patients with stable angina undergoing percutaneous coronary intervention [59].
Coronary Spasm
Nicorandil is particularly useful in the treatment of coronary spasm, which may arise due to variant (Prinzmetal’s) angina, mixed (variable–threshold) angina, or in the context of an acute coronary syndrome [60]. Its dual mode of action confer efficacy for both epicardial artery and microvascular spasm. Moreover, patients with variant angina show greater vasodilatory response to nicorandil, than those with effort-induced or post-myocardial infarction symptoms [61].
In a study of 32 patients with variant angina performed in 1987, Kisheda et al. found that nicorandil significantly reduced the frequency of angina episodes and ST-segment changes compared to placebo after 3 days [62]. Angina attacks disappeared completely in 24 of the 32 patients, and overall the average number of angina episodes decreased from 3.6 ± 0.4 per day to 0.7 ± 0.2 per day in the study group, and subsequently increased after stopping nicorandil. Nicorandil can also reverse coronary spasm induced by ergonovine [63], and is at least as effective in preventing coronary spasm as nifedipine [64]. Anecdotal evidence from several case reports suggests that intracoronary nicorandil may be more effective than other agents for relieving acute epicardial artery spasm [65, 66], and for improving coronary slow-flow phenomenon due to microvascular spasm [67–69].
Cardio-Protection
The cardio-protective role of nicorandil in the clinical setting was shown in the IONA (Impact of Nicorandil in Angina) and JCAD (Japanese Coronary Artery Disease) studies [70, 71]. In IONA, 5,126 patients with stable angina were randomized to receive 20 mg of nicorandil or placebo; this showed a significant reduction in cardiac events (composite end-point of death from coronary heart disease, nonfatal myocardial infarction, or hospital readmission for chest pain) in the study group after a mean follow-up of 1.6 years (hazard ratio 0.83, p = 0.014) [70]. The results of IONA were reported in 2002, and subsequent sub-group analyses showed no evidence of any qualitative or quantitative interactions between nicorandil treatment benefit and subgroup status (including age, sex, risk factors, previous cardiac history, angina status, other medications and overall assessment of risk) [72].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


