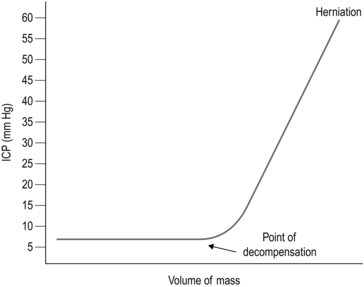
35 1. Damage to the brain will result in disability at remote sites which is not always proportional to the extent of brain damage: a massive lesion in a non-eloquent area such as the right frontal lobe can cause virtually no disability whereas a lesion no larger than a few millimetres in the internal capsule or brainstem can cause hemiplegia. Modern neurosurgical approaches make ingenious use of corridors through the skull base or non-eloquent areas of the brain: it is always preferable to remove more skull and retract the brain less. 2. Although the brain is 2% of the body weight, it receives 15% of cardiac output. It depends on this massive blood supply to support its extremely high metabolism and tolerates any disruption poorly. It can also bleed catastrophically and the usual haemostatic measures of applying firm pressure and ligating vessels cannot be used due to the risk of causing neurological injury. 3. Emergency neurosurgery generally involves removing pressure from some point of the neuroaxis to prevent extension of injury. This can ameliorate secondary injury, and if the primary injury can be survived this allows some chance of recovery: 1. The head is vulnerable to injury due to its position and its weight. Under normal conditions it is supported by powerful muscles in the neck, but in high-speed deceleration its weight results in massive forces acting on it about the moment of the top of the chest. Most assaults include blows to the head. 2. Patients with a severe head injury frequently have neck injuries and this must be assumed and the cervical spine protected unless the patient is fully conscious and the neck can be cleared of injury by clinical examination according to ATLS protocol. 3. Patients with severe head injury frequently require immediate surgery to save their life; however, adequate resuscitation is of greater importance: operating on a hypoxic hypotensive patient with an unprotected fractured cervical spine is far more likely to result in the patient’s death than delaying whilst they are resuscitated: an intubated, oxygenated patient with a sustainable blood pressure and a protected cervical spine is in a far better condition to tolerate a trauma craniotomy. Specific management must be preceded by adequate resuscitation. Intracranial pressure (ICP) is defined as the pressure of CSF in the frontal horn of the lateral ventricle. The relationship between ICP and the volume of an expanding mass lesion within the brain is given by the Monro-Kellie doctrine (Fig. 35.1). This divides the intracranial contents into compartments: brain, CSF, venous blood, and arterial blood. There may be an extra compartment in the form of a mass lesion such as haematoma. The doctrine states that since the intracranial volume is fixed, an increase in one compartment must be compensated by a decrease in another or ICP will rise. In effect this means that normal ICP can be maintained with an expanding haematoma by displacing CSF and venous blood (the total volume is approximately 100 ml). Thereafter, ICP will rise steeply, clinically manifest by a sudden and often catastrophic deterioration in the patient’s conscious level. This explains the lucid interval seen in extradural haematoma (see below). Subsequent decrease in the volume of the arterial blood compartment will produce ischaemia, with further injury and swelling. As a haematoma expands, the brain is displaced towards the foramen magnum, and pressure builds up in the conical posterior fossa causing the brainstem to herneate through the foramen magnum, compressing the respiratory and cardiac centres in the medulla – this is ‘coning’. Ischaemia and herniation are the mechanisms of death from raised ICP. The intracranial pressure must be reduced immediately: 1. Secure the airway with endotracheal intubation and hyperventilate to a PaCO2 of 4–4.5 KPa, or double the minute ventilation if ABGs are not immediately available. This causes a reduction in PaCO2 resulting in reduced cerebral blood flow. Although this is ultimately bad for the brain, it results in an immediate drop in intracerebral pressure and can save the patient’s life. 2. Sit the patient up to between 30 and 45 degrees. This can still be accomplished with a fractured cervical spine. Make sure that nothing is compressing the neck veins: if blood cannot leave the head venous congestion will needlessly raise ICP. Cervical collars should be removed as soon as the patient has been transferred to a trolley. Bolsters either side of the head, with tape across forehead and chin, anchored either side of the trolley, will hold the neck still and prevent unnecessary compromise of the neck veins. Collars should only ever be used for transferring patients. 3. Administer Mannitol: accurate dose calculations are time consuming and based on a (usually wildly inaccurate) estimate of the patient’s weight. Give 200 ml of 20% over 20 minutes (or 400 ml of 10%, etc.). This dose is easy to remember when the patient’s pupil has just fixed. Insert a urinary catheter since a massive diuresis will occur within 15 minutes. 4. As soon as the patient is stable obtain cross sectional imaging, if available. There is no place for skull X-rays when CT scanning is available. A typical anaesthetic for elective craniotomy may be given with the following: 1. I.V. induction of anaesthesia with Propofol with or without a strong opiate (fentanyl/alfentanil/remifentanil). 2. A muscle relaxant is given and the trachea is intubated with a reinforced/armoured endotracheal tube. 3. The response to laryngoscopy may be obtunded using adjuvant drugs. 4. Anaesthesia is maintained with either ≈ 1 MAC of volatile agent ± analgesia or total intravenous anaesthesia (TIVA). A non-depolarizing muscle relaxant is used. 5. Normotension and normocarbia are usually maintained, but certain surgical procedures may require relative hypotension. 6. At the end of the operation, if the patient is to be woken up, the muscle relaxant is reversed and the volatile agent/TIVA is turned off. Once the patient is able to breathe they are allowed to wake up. Analgesia is given as required. Some patients are electively intubated and ventilated after surgery to protect the brain using anaesthesia or maintain strict blood pressure control. 1. An unsutured small scalp laceration can bleed profusely, so arrest bleeding from the scalp as soon as is practicable after injury, using a temporary single layer of through-and-through sutures. 2. If the patient has been struck on the head with a heavy object, X-ray the skull to ensure that the laceration does not cover a depressed skull fracture. 1. Shave adequately and closely round the laceration before exploring it. 2. The scalp is vascular, heals well and seldom gets infected, so do not excise the contused edges of the laceration too enthusiastically which will produce a scalp defect requiring apposition of the edges under tension. 3. If the galea has been breached, always close the scalp laceration in two layers, one for the galea and a second through-and-through skin layer, as previously described. 4. If possible, transfer a patient with severe scalp loss to a special unit where plastic and neurosurgical facilities are available. 1. The purpose of elevating a depressed skull fracture is to reduce the risk of infection, so elevate only compound depressed skull fractures. 2. Leave alone depressed fractures with intact overlying scalp. Occasionally, the dislocation of the skull contour may be so great that elevation is required for cosmetic reasons, but these more severe injuries are invariably compound, so that the customary indication for operation is also present. 1. Excise the overlying scalp laceration if it is badly contused. Extend the incision to give access to the whole depressed area. Scrape the scalp off the underlying bone and hold the incision wide open with self-retaining retractors. 2. Clear the pericranium away with a periosteal elevator to reveal the whole depressed area. 3. The inner table will have been driven in over a much wider area than the visible area of depressed outer table. Make a single burr hole just outside the edge of the visibly depressed region in order to expose dura not involved in the depression. 1. Insert a periosteal elevator into the burr hole, slide it gently between the bone and the dura and ease out the depressed fragments so that the dura beneath them is fully exposed. Remove dirt, debris and any small flakes of bone from the wound and send them for bacteriological culture. 2. If the dura is intact, do not open it. If it is lacerated, carefully extend the laceration to inspect the brain beneath. If the brain surface is torn, probe gently in the tear for any in-driven debris and bone and remove them. 3. Remove pulped and clearly necrotic brain tissue by a combination of gentle suction and irrigation with 0.9% saline at body temperature. 4. Coagulate bleeding points in the brain with low-intensity diathermy coagulation, and diffuse oozing by applying patches of surgical cellulose compressed into place beneath lintine strips. 5. If the depressed bone fragments have been driven through the dura, their removal may tear large cerebral vessels as the fragments are extracted. A large cerebral vessel, not visible on the brain surface, may be picked up and held in the tip of a fine sucker under fairly strong suction while it is coagulated with diathermy or occluded with a metal clip. 6. If a sinus is torn, do not try to close it with sutures. Reduce the pressure in the sinus by tilting the patient feet-down, then cover the sinus with several layers of surgical cellulose and hold them firmly in place under lintine strips for 5–10 minutes. When you release the pressure and remove the lintine, the bleeding should not recur. Do not now disturb the surgical cellulose. 1. Before closing, irrigate the whole wound with hydrogen peroxide solution and 20 ml of 0.9% saline containing 20 000 units of penicillin and 50 mg of streptomycin. 2. Close the dura with interrupted 3/0 silk sutures. Cover any gaps in the dura with two layers of surgical cellulose. 3. Unless the wound has been neglected and is obviously infected, replace the removed bone fragments on the dura to fill in the skull defect. Scrub them thoroughly in aqueous Savlon before replacing them.
Neurosurgery
THE ETHOS OF NEUROSURGERY
 Primary injury relates to structural neurological damage caused by transfer of kinetic energy, pressure, shearing, etc. This injury is static, but may be overwhelming; for example, a high-velocity bullet wound.
Primary injury relates to structural neurological damage caused by transfer of kinetic energy, pressure, shearing, etc. This injury is static, but may be overwhelming; for example, a high-velocity bullet wound.
 Secondary injury arises from local and systemic events that follow. There will be a local inflammatory cascade and subsequent oedema. In the enclosed space of the skull and spine this will create raised pressure resulting in impaired perfusion. Systemic hypotension and hypoxia will be equally deleterious.
Secondary injury arises from local and systemic events that follow. There will be a local inflammatory cascade and subsequent oedema. In the enclosed space of the skull and spine this will create raised pressure resulting in impaired perfusion. Systemic hypotension and hypoxia will be equally deleterious.
TRAUMA
INTRACRANIAL PRESSURE
INITIAL MANAGEMENT OF THE OBTUNDED HEAD INJURED PATIENT
ANAESTHETIC CONSIDERATIONS
Specific considerations for anaesthetic drugs
 Induction agents: propofol can cause hypotension (given slowly to minimize effect). Thiopentone may be useful to reduce CMRO2 (cerebral metabolic rate of oxygen) and seizure activity.
Induction agents: propofol can cause hypotension (given slowly to minimize effect). Thiopentone may be useful to reduce CMRO2 (cerebral metabolic rate of oxygen) and seizure activity.
 Narcotics/opiates: (fentanyl, morphine and alfentanyl) may reduce CBF and ICP, but can raise ICP in patients with brain trauma.
Narcotics/opiates: (fentanyl, morphine and alfentanyl) may reduce CBF and ICP, but can raise ICP in patients with brain trauma.
 TIVA: remifentanil is usually given as an infusion.
TIVA: remifentanil is usually given as an infusion.
 Inhalational agents: isoflurane, desflurane or sevoflurane are preferred. Halothane, enflurane and nitrous oxide all affect cerebral autoregulation and can raise ICP.
Inhalational agents: isoflurane, desflurane or sevoflurane are preferred. Halothane, enflurane and nitrous oxide all affect cerebral autoregulation and can raise ICP.
 Paralysis: non-depolarizing drugs are preferred (e.g. atracurium, vecuronium, rocuronium).
Paralysis: non-depolarizing drugs are preferred (e.g. atracurium, vecuronium, rocuronium).
SCALP LACERATIONS
Appraise
Action
DEPRESSED SKULL FRACTURE
Appraise
Access
Action
Closure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurosurgery