and Ronak K. Kapadia1
(1)
Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Brain Tumors
Pathophysiology
CLASSIFICATION BY HISTOLOGY
neuroepithelial
gliomas
astrocytoma (30%)—pilocytic astrocytoma (WHO Grade I), diffuse astrocytoma (WHO Grade II), anaplastic (WHO Grade III), glioblastoma (WHO Grade IV, 20% of all brain tumors)
oligodendroglioma (4%)—low grade (WHO Grade II), anaplastic (WHO Grade III), mixed; 50–80% have 1p19q co-deletion which is associated with improved survival and favorable response to chemotherapy and/or radiation
ependymoma (2%) (WHO Grade II or III)
choroid plexus tumors (WHO Grade II or III)
neuronal and mixed neuronal–glial tumors (WHO Grade I or II)
pineal parenchymal tumors
embryonal tumors (1.7%)—medulloblastoma (WHO Grade IV), pineoblastoma, neuroblastoma, ependymoblastoma
cranial/spinal nerves—schwannoma, neuro-fibroma, malignant peripheral nerve sheath tumor (malignant schwannoma, 8%)
meninges
benign meningioma (30%) (WHO Grade I)
atypical meningioma (WHO Grade II)
anaplastic meningioma (WHO Grade III)
malignant neoplasms—hemangiopericytoma, chondrosarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma, meningeal sarcomatosis
primary melanocytic lesions—diffuse melanosis, melanocytoma, malignant melanoma
lymphoma (3%)—malignant lymphomas, plasmacytoma, granulocytic sarcoma
germ cell—germinoma, embryonal carcinoma, choriocarcinoma, teratoma
cysts and tumor like—Rathke cleft cyst, epidermoid cyst, dermoid cyst, colloid cyst
sellar region—pituitary adenoma (9–13%), pituitary carcinoma, craniopharyngioma (2–5%)
local extension from regional tumors—paraganglioma, chordoma, chondrosarcoma
metastatic tumors
RISK FACTORS
family history
environmental—radiation (meningioma, glioma), vinyl chloride (glioma)
diseases—HIV (CNS lymphoma), familial adenomatous polyposis (medulloblastoma), Li–Fraumeni syndrome (astrocytomas), Turcot’s syndrome (medulloblastoma, glioblastoma), neurofibromatosis (astrocytomas, nerve sheath tumours)
GLIOBLASTOMA DEVELOPMENT
—in elderly patients, more likely de novo (primary GBM). In younger patients, more likely evolved from low-grade glioma (secondary GBM) with stepwise mutation
MANAGEMENT IN GLIOBLASTOMA
—epigenetic silencing with methylation of MGMT (O6-methylguanine–methyltransferase) DNA-repair gene is both prognostic and predictive of better outcomes. Inactivation of MGMT prevents it from repairing the damage caused by alkylating agents, thus contributing to increased effectiveness of treatment
MASS EFFECT
—tumors → vasogenic edema → direct compression of neurons causing demyelination and necrosis and specific neurological symptoms based on anatomical location. Also increases intracranial pressure causing headache, nausea and vomiting, papilledema, third nerve palsy, and herniation syndromes. Hydrocephalus may also occur with obstruction of third or fourth ventricle due to posterior fossa tumors
Related Topics
CNS lymphoma (p. 196)
Seizures (p. 350)
Headaches (p. 355)
Clinical Features
SYMPTOMS
—headache (70%), seizure (50%, more with low-grade tumors), focal neurological deficits (motor, sensory, more with high-grade tumors), cognitive dysfunction, visual spatial dysfunction, aphasia, N&V, altered level of consciousness
SIGNS
—cranial nerve examination, with particular attention to fundoscopy and visual fields (driving), cognitive assessment with MMSE or Montreal Cognitive Assessment (MoCA), speech, motor, sensory, gait, cerebellum, pronator drift, Romberg sign
Investigations
BASIC
labs—CBCD, lytes, urea, Cr, AST, ALT, ALP, bilirubin, INR, PTT, albumin
imaging—MRI head, CT head
biopsy—open biopsy, stereotactic biopsy
SPECIAL
MR spectroscopy—N-acetylaspartate, choline, lactate
functional MR—blood flow
Prognostic Issues
PROGNOSIS FOR LOW-GRADE GLIOMAS
—median survival 7–8 years, 5-year survival 50–94%; median time to recurrence 4.5 years, median survival from recurrence 12 months
PROGNOSIS FOR GLIOBLASTOMA
—median survival 14 weeks with observation only, 20 weeks with resection, 36 weeks with radiation added, and 40–60 weeks with chemotherapy added
PROGNOSTIC FACTORS FOR ANAPLASTIC ASTROCYTOMA AND GLIOBLASTOMA
—older age, poor Karnofsky performance status, degree of excision, neurologic deficits
MEDIAN SURVIVALS FOR OLIGODENDROGLIOMA
Oligodendroglioma | 1p19q deletion | No1p19q deletion |
|---|---|---|
Low grade | 15 years | 5 years |
High grade | 5–10 years | 2 years |
Management
SYMPTOM CONTROL
—seizure control (phenytoin, levetiracetam, carbamazepine, lamotrigine, clobazam, valproate, topiramate), steroids may be used short term for cerebral edema with symptoms such as headaches, neurological deficits
TUMOR CONTROL
astrocytoma
low grade (grade II)—maximal surgical debulking. Upfront radiation improves progression-free survival but not overall survival. Thus, it may be delayed in patients who are asymptomatic
anaplastic (grade III)—maximal surgical debulking, followed by radiation ± chemotherapy (PCV, temozolomide)
glioblastoma (grade IV)—maximal surgical debulking, concurrent chemoradiation with temozolomide ×6 weeks, followed by 4-week break and then adjuvant temozolomide d1–5 q28d ×6
low–grade oligodendroglioma
with 1 p 19 q deletion—resection. Chemotherapy at progression to delay radiation is an option
without 1 p 19 q deletion—resection. Radiation may be delayed until progression or symptoms
high–grade oligodendroglioma
with 1 p 19 q deletion—resection ± chemotherapy ± radiation
without 1 p 19 q deletion—resection, RT alone or concurrent chemoradiation with temozolomide ×6 weeks, followed by 4-week break and then adjuvant temozolomide d1–5 q28d ×6
salvage chemotherapy for gliomas—nitrosoureas, bevacizumab, etoposide, carboplatin, procarbazine
ependymoma—resection ± radiation. Palliative chemotherapy may be provided with recurrence
primary neuroectodermal tumors (medulloblastoma, supratentorial, pineoblastoma)—resection plus craniospinal radiation for low risk tumors may be curative. Add adjuvant chemotherapy (cisplatin, etoposide, cyclophosphamide or lomustine and vincristine) for high-risk tumors
meningioma—observation if asymptomatic and no mass effect. Otherwise, resection or radiation if surgery not possible
DRIVING
—the key factors that affect driving include seizures, visual fields, motor deficits, and cognition
Treatment Issues
SIDE EFFECTS OF BRAIN IRRADIATION
radionecrosis—contrast-enhancing focal lesion may be difficult to differentiate from recurrent brain tumor. Supportive measures
radiation–induced leukoencephalopathy—occurs months to years later. Symptoms may include gait ataxia, urinary incontinence, and dementia
radiation myelopathy—associated with accumulative radiation dose to the spinal cord, peaking at 1 and 2 years. Symptoms may include Lhermitte’s sign, paresthesias (pain and temperature) with progressive loss of cord function over 6 months. Supportive measures only
Specific Entities
HERNIATION SYNDROMES
transtentorial—symmetric downward displacement of the hemispheres, causing impaction of the diencephalon and midbrain into the tentorial notch → rostrocaudal deterioration with decorticate evolving to decerebrate posturing
uncal—temporal lobe and uncus shift medially into the tentorial notch, causing compression of third nerve (pupillary dilation, eye deviation ‘down and out’) and contralateral cerebral peduncle (ipsilateral hemiparesis, false localizing sign)
tonsillar—cerebellar tonsils downward into the foramen magnum compresses the medulla and upper spinal cord, resulting in rapid failure of vital functions
BRAIN METASTASES
pathophysiology—occurs in 20–40% of patients, most commonly from lung, breast, melanoma, renal cell, and gastrointestinal cancers. About 10× more frequent than primary brain tumors. Found in cerebral hemispheres, cerebellum, and brainstem 80%, 15% and 5% of the time
treatment—surgery plus radiation offers survival advantage over radiation alone, although <50% of brain metastases are resectable. Radiation reduces recurrence but does not improve survival
LEPTOMENINGEAL CARCINOMATOSIS
pathophysiology—occurs in 5% of patients, most commonly from leukemias, non-Hodgkin’s lymphoma, and solid tumours (lung, breast, and melanoma)
diagnosis—CSF analysis for cytologic confirmation (multiple taps often necessary). MRI spine may also be helpful
treatment—median survival 4–6 weeks without treatment and may improve to 3–6 months with intrathecal therapy (methotrexate, cytarabine, thiotepa). Necrotizing leukoencephalopathy may develop months after in those who survived, particularly after combined methotrexate and radiation administration
Acute Stroke Syndromes
NEJM 2007 357:6
NEJM 2008 359:13
AHA/ASA Stroke Guidelines 2009
Differential Diagnosis
ISCHEMIC STROKE
thrombotic/intrinsic vessel disease—atherosclerosis, vasculitis, vasospasm, dissection, compression, fibromuscular, hypercoagulable state
embolic/remote origin—cardiogenic, artery, septic, air, fat, paradoxical (from VTE)
global ischemia—MI, VT
HEMORRHAGIC STROKE
intracerebral vessel rupture—hypertension, trauma, bleeding diatheses, amyloid angiopathy, illicit drug use, vascular malformation
subarachnoid vessel rupture—aneurysm rupture, vascular malformation, bleeding diatheses, trauma, amyloid angiopathy, illicit drug use (cocaine)
STROKE MIMICS
(usually global rather than focal neurological symptoms) ★DIMS★
D rug intoxication / Withdrawal
I nfections
I nsanity—conversion disorder
M etabolic—hypoglycemia, renal failure, hepatic failure, hypoxia/hypercarbia, endocrine disorders (thyrotoxicosis, myxedema, adrenal insufficiency)
M igraines
S yncope
S eizures—Todd’s paralysis
S tructural—trauma, tumors, subdural hemorrhage
Pathophysiology
FIVE QUESTIONS
1.
Is the patient stable?
2.
Is this a stroke?
3.
Where is the stroke? Symptoms/signs, CT head
4.
What kind of stroke? Ischemic (thrombotic, embolic, global ischemic), hemorrhagic (intracerebral, subarachnoid)
5.
How to manage the patient? Thrombolytics?
PATHOPHYSIOLOGIC STROKE CLASSIFICATION
thrombotic stroke
1.
large vessel stroke—most commonly due to atherothrombosis. Found at bifurcation of common carotid artery, siphon portion of common carotid artery, middle cerebral artery stem, intracranial vertebral arteries proximal to middle basilar artery, origin of vertebral arteries
2.
small vessel stroke (lacunar/penetrating vessels)—most commonly due to lipohyalinotic occlusion related to hypertension and occasionally atheroma at the origin of vessels. Found at penetrating branches of the anterior, middle, and posterior cerebral and basilar arteries
cardioaortic embolic stroke
1.
cardiac sources definite (anticoagulant or antithrombotic therapy generally used)—LV thrombus, LA thrombus, rheumatic valve disease, artificial valve (mechanical, bioprosthetic), AF
2.
cardiac sources definite (anticoagulation hazardous)—bacterial endocarditis, atrial myxoma
3.
cardiac sources possible—mitral annular calcification, left ventricular dysfunction, status post-MI, LA spontaneous echo contrast, PFO, ASD, mitral valve strands
4.
unknown source embolic stroke
5.
others—dissection, moyamoya, primary thrombosis, cerebral mass
RISK FACTORS FOR STROKE
thrombotic—age, smoking, diabetes, dyslipidemia, hypertension, family history, male, history of TIA
embolic—smoking, diabetes, dyslipidemia, hypertension, family history, male, history of heart disease (valvular, AF, endocarditis)
ICH—hypertension, trauma, bleeding diatheses, illicit drugs, vascular malformations, blacks, Asians
SAH—illicit drugs, bleeding diatheses
COMPLICATIONS OF STROKE
—about 25% of patients can worsen during the first 24–48 h after stroke
neurologic—cerebral edema, seizures, hemorrhagic transformation of infarction with or without hematoma, neurological deficits
non–neurologic—myocardial infarction, arrhythmia, aspiration, pneumonia, DVT, pulmonary embolism, malnutrition, pressure sores, orthopedic complications, contractures
MAP OF MOTOR/SENSORY CORTEX
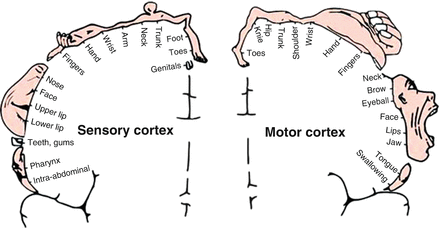
Reprinted with permission from Humana Press
Clinical Features
TRANSIENT ISCHEMIC ATTACK
—defined as an ischemic episode with full recovery within 24 h. Most TIAs last <5 min, while most ischemic attacks >1 h are associated with infarction. Risk of stroke in patients with TIA is 5% within 2 days and 10% within 90 days
PREDICTION OF STROKE RISK AFTER TIA
★ABCD2★ criteria
Age—1 = age >60 years,
Blood pressure—1 = hypertension at the acute evaluation (>140/90 mmHg)
Clinical features—2 = unilateral weakness, 1 = speech disturbance without weakness
Duration of symptom—1 = 10–59 min, 2= > 60 min
Diabetes—1 = present
interpretation
low risk (scores 0–3) = risk of stroke 1.0% at 2 days. Hospital observation may not be necessary without another indication such as new-onset atrial fibrillation
moderate risk (scores 4–5) = risk of stroke 4.1% at 2 days. Hospital observation justified in most situations
high risk (scores 6–7) = risk of stroke 8.1% at 2 days. Hospital observation recommended
CLINICAL STROKE CLASSIFICATION
anterior cerebral artery (embolic > thrombotic)—motor and sensory deficit (leg > arm, face), frontal release signs (grasp, snout, root, and suckling reflexes), abulia, paratonic rigidity, gait apraxia, personality Δ
middle cerebral artery (left dominant hemispheric, embolic > thrombotic)—aphasia, right hemiparesis, and sensory deficit (face, arm > leg), may be complete hemiplegia if internal capsule involved, right spatial neglect, right homonymous hemianopia, impaired right conjugate gaze
middle cerebral artery (right non-dominant hemispheric, embolic > thrombotic)—anosognosia, left motor and sensory deficit (face, arm > leg), left spatial neglect, left homonymous hemianopia, impaired left conjugate gaze
deep (subcortical/lacunar) hemisphere or brainstem (small artery infarct)—hemiparesis (pure motor stroke); sensory loss (pure sensory stroke); dysarthria and clumsy hand syndrome; ataxic hemiparesis. No abnormalities of cognition, language, or vision
posterior cerebral artery (embolic > thrombotic)—homonymous hemianopia with macular sparing, alexia without agraphia (dominant hemisphere), visual hallucinations, visual perseverations (calcarine cortex), choreoathetosis, spontaneous pain (thalamus), third nerve palsy, paresis of vertical eye movement, sensory loss, motor deficit (cerebral peduncle, midbrain)
vertebrobasilar artery (brainstem, embolic = thrombotic)—motor or sensory loss in ALL 4 limbs; crossed signs (ipsilateral cranial nerve palsy with contralateral motor/sensory deficit), dysconjugate gaze, nystagmus, ataxia, dysarthria, dysphagia
cerebellum—ipsilateral limb ataxia, gait ataxia
internal carotid artery (thrombotic > embolic)—progressive or stuttering onset of MCA syndrome, occasionally ACA syndrome as well
RATIONAL CLINICAL EXAMINATION SERIES: IS THIS PATIENT HAVING A STROKE? PRE-TEST LIKELIHOOD
—probability of a stroke among patients with neurologically relevant symptoms is 10%
LR+ | LR– | |
|---|---|---|
Pre–hospital assessment | ||
Presence of any one of acute facial paresis, arm drift, or abnormal speech | 5.5 | 0.39 |
In–hospital clinical assessment | LR+ | Prob. stroke |
Focal neurological deficit, persistent neurological deficit, acute onset during prior week, no history of head trauma | ||
0 factor | 0.14 | 1.5% |
1–3 factors | – | ≥10% |
4 factors | 40 | 80% |
NIH STROKE SCALE—level of consciousness (0 = alert, 1 = not alert, 2 = obtunded, 3 = unresponsive), level of consciousness questions (0 = answers both correctly, 1 = answers one correctly, 2 = answers neither correctly), level of consciousness commands (0 = performs both tasks correctly, 1 = performs one task correctly, 2 = performs neither task), gaze (0 = normal, 1 = partial gaze palsy, 2 = total gaze palsy), visual fields (0 = no visual loss, 1 = partial hemianopsia, 2 = complete hemianopsia, 3 = bilateral hemianopsia), facial palsy (0 = normal, 1 = minor paralysis, 2 = partial paralysis, 3 = complete paralysis), left motor arm (0 = no drift, 1 = drift before 5 s, 2 = falls before 10 s, 3 = no effort against gravity, 4 = no movement), right motor arm (0 = no drift, 1 = drift before 5 s, 2 = falls before 10 s, 3 = no effort against gravity, 4 = no movement), left motor leg (0 = no drift, 1 = drift before 5 s, 2 = falls before 5 s, 3 = no effort against gravity, 4 = no movement), right motor leg (0 = no drift, 1 = drift before 5 s, 2 = falls before 5 s, 3 = no effort against gravity, 4 = no movement), ataxia (0 = absent, 1 = one limb, 2 = two limbs), sensory (0 = normal, 1 = mild loss, 2 = severe loss), language (0 = normal, 1 = mild aphasia, 2 = severe aphasia, 3 = mute or global aphasia), dysarthria (0 = normal, 1 = mild, 2 = severe), extinction/inattention (0 = normal, 1 = mild, 2 = severe)
Refer to www.ninds.nih.gov/doctors/nih_stroke_scale.pdf for online version of NIH Stroke Scale
APPROACH—onset of symptoms → prehospital assessment → in-hospital assessment → if likely stroke, assess with NIH stroke score, perform neuroimaging and laboratory tests to exclude stroke mimics → begin stroke treatment. “The accurate determination of stroke subtype requires neuroimaging to distinguish ischemic from hemorrhagic stroke. Early mortality increases among those with any one of impaired consciousness, hemiplegia, and conjugate gaze palsy (LR+ 1.8, LR 0.36)”
JAMA 2005 293:19
Related Topics
CT Head (p. 378)
Dysphagia (p. 124)
RATIONAL CLINICAL EXAMINATION SERIES: DOES THIS PATIENT HAVE A CLINICALLY IMPORTANT CAROTID BRUIT?
Sens | Spc | LR+ | |
|---|---|---|---|
Ability of carotid bruits to indicate carotid stenosis in symptomatic patients | |||
TIA patients with >50% stenosis | 29% | 88% | 2.4 |
Anterior circulation TIA patients with 75–99% stenosis | 76% | 76% | 3.2 |
Anterior circulation TIA patients with 70–99% stenosis | 62% | 61% | 1.6 |
Ability of carotid bruit to predict carotid stenosis in asymptomatic patients | |||
Bruit predicting carotid stenosis (70–99%) | – | – | 6.0 |
APPROACH—“although the presence of a carotid bruit in a patient with carotid-territory TIA/stroke increases the probability that the underlying stenosis is high grade (and therefore amenable to endarterectomy), the accuracy of this physical finding is low. Accordingly, carotid bruit cannot be used to rule in or rule out surgically amenable carotid artery stenosis in symptomatic patients. Asymptomatic preoperative bruits are not predictive of increased risk of perioperative stroke. However, they may be harbingers of transient postoperative cognitive and behavioral abnormalities”
JAMA 1993 270:23
The Rational Clinical Examination. McGraw-Hill, 2009.
CLINICAL CLUES TO DIAGNOSIS
thrombotic—stuttering progression with periods of improvement. Lacunes develop over hours or at most a few days; large artery ischemia may evolve over longer periods. May have neck bruit or prior TIAs
embolic—sudden onset with deficit maximal at onset. Clinical findings may improve quickly. Can be precipitated by getting up at night to urinate, or sudden coughing or sneezing
ICH—gradual progression over minutes to hours. May be precipitated by sex or physical activities
SAH—abrupt onset, thunderclap, severe headache, focal brain dysfunction less common. May be precipitated by sex or other physical activity
RATIONAL CLINICAL EXAMINATION SERIES: DOES THIS PATIENT HAVE A HEMORRHAGIC STROKE?
In patients for whom clinical diagnosis of stroke has already been made (see JAMA 2005;293), differentiation of ischemic vs. hemorrhagic subtypes guides treatment
LR+ | LR– | |
|---|---|---|
Risk factors | ||
Coronary artery disease | 0.44 | 1.1 |
Atrial fibrillation | 0.44 | 1.1 |
Peripheral arterial disease | 0.41 | 1.1 |
Prior TIA | 0.34 | 1.2 |
Symptoms | ||
Seizures with neuro deficit | 4.7 | 0.93 |
Vomiting | 3.0 | 0.73 |
Headache | 2.9 | 0.66 |
Loss of consciousness | 2.6 | 0.65 |
Physical signs | ||
Coma | 6.2 | – |
Neck stiffness | 5.0 | 0.83 |
DBP >110 mmHg | 4.3 | 0.59 |
Cervical bruit | 0.12 | 1.1 |
Laboratory findings | ||
Xanthochromia in CSF | 15 | 0.31 |
Atrial fibrillation on EKG | 0.19 | 1.2 |
Clinical impression and stroke scores | ||
Clinician’s impression hemorrhage is most likely Dx | 6.2 | 0.28 |
Siriraj Stroke Score >1 (hemorrhage) | 5.7 | – |
Siriraj Stroke Score < −1 (infarction) | 0.3 | – |
Besson score ≥1 (hemorrhage) | 1.4 | – |
Besson score <1 (infarction) | 0.2 | – |
APPROACH—among stroke patients, the presence of several clinical findings such as headaches, vomiting, severe hypertension, neck stiffness, and coma increase the probability of hemorrhagic stroke. However, because these findings only have low to moderate diagnostic accuracy, neuroimaging is recommended for definitive diagnosis. Xanthochromia has high diagnostic performance but requires an invasive procedure
JAMA 2010 303:22
Investigations
BASIC
labs—CBCD, lytes, urea, Cr, glucose, troponin, CK, PTT, INR, AST, ALT, ALP, bilirubin, total cholesterol, TGL, LDL, HDL, homocysteine, ESR
imaging—CT head without contrast, MRI head (more sensitive than CT head in detecting acute ischemic stroke), angiogram (CT, MR, contrast), carotid dopplers, echocardiogram (TEE > TTE)
SPECIAL
ecg—ST depression, QT prolongation, inverted T, prominent U waves
holter monitor—evaluation for occult atrial fibrillation
eeg—if seizures
toxicology screen
Diagnostic and Prognostic Issues
DOMINANT HEMISPHERE
—the left hemisphere is dominant (language functions) in 95% of right-handed and 70% of left-handed individuals
CT HEAD
—gold standard, but relatively insensitive in detecting acute and small cortical or subcortical infarctions, especially in the posterior fossa. Critical for excluding hemorrhagic disease. Early signs (within 6 h) of MCA infarction include hyperdense middle cerebral artery sign (thrombus or embolus in first portion of MCA), loss of gray–white differentiation in the cortical ribbon (especially at the lateral margins of the insula), or lentiform nucleus and sulcal effacement. Hypodense lesions may not appear until after 24 h. They become more hypodense overtime
MORTALITY RATE
—30-day mortality post-ischemic stroke is 10–17%
PROGNOSTIC MARKERS
—age, degree of neurological deficit (NIH stroke scale), extent of stroke on CT, fever
Management
ACUTE
—ABC, O2, IV, do not treat blood pressure unless extreme to avoid further ischemic insult (hypertensive encephalopathy or >220/120 mmHg, then labetalol 10 mg IV q5–10 min or 200–400 mg PO BID until BP <185/110 mmHg); for patients eligible for thrombolysis, treat if BP ≥185/110. Thrombolytics (if within 4.5 h of onset of ischemic stroke, see below; alteplase 0.9 mg/kg IV, maximum 90 mg). Anticoagulation is not indicated unless embolic stroke with obvious cardiac source (e.g. atrial fibrillation). ASA 81–325 mg PO daily (if thrombolytics given, may start ASA after first 24 h. For long-term secondary prophylaxis, consider clopidogrel or dipyridamole if cannot tolerate ASA). If SAH, consider nimodipine. Neurology or neurosurgery consult. Early mobilization/rehabilitation with multi-disciplinary team management (e.g. swallowing assessment prior to initiating diet, physiotherapy, occupational therapy). Monitor complications and treat other cardiovascular risk factors
Treatment Issues
THROMBOLYSIS
inclusion—clinical diagnosis of ischemic stroke, age 18–80 years, onset of symptoms within 4.5 h, measurable neurological deficit, stroke symptoms present for at least 30 min with no significant improvement before treatment
exclusion—historical (time of symptom onset unknown, prior history of ICH, stroke/head trauma <3 months, MI <3 months, major surgery/trauma <14 days, GI/GU bleed <21 days, arterial puncture in non-compressible site <7 days, combination of previous stroke and DM, oral anticoagulant treatment, coagulopathy), clinical (rapidly improving stroke symptoms, minor/isolated symptoms, seizure at onset of stroke with residual impairment secondary to postictal phenomenon, suspicion of SAH, acute MI/post-MI pericarditis, persistent hypertension ≥185/110), labs (platelet <100 × 109/L, glucose <2.8 mM [50 mg/dL], ↑ PTT), CT head (hemorrhage, major early infarct signs), severe stroke as assessed clinically (NIH score >25) or radiographically (stroke involving >1/3 of cerebral hemisphere)
outcome—among patients receiving thrombolysis within 3 h of onset, favorable outcomes in 31–50% of treated patients compared to 20–38% of nontreated patients at 3 months and 1 year. Patients benefit more if treated early (<90 min) but benefit extends out to 6 h. Major risk is symptomatic brain hemorrhage (3–5%). However, mortality rate is similar between the two groups at 3 months and 1 year. Thrombolysis administered between 3 and 4.5 h after symptom onset associated with favorable outcome in 52.4% compared to 45.2% in non-treated patients, with an increased risk of intracranial hemorrhage, but no effect on mortality
RELATIVE RISK REDUCTION FOR ISCHEMIC STROKE/TIA
Condition | Primary prophylaxis | Secondary prophylaxis |
|---|---|---|
Hypertension | Anti-HTN 20% | Anti-HTN 28% |
Hyperlipidemia | Statins | Statins |
Atrial fibrillation | ASA 20–30% | ASA 20–30% |
Coumadin 60% | Coumadin 60% | |
Post-MI | ASA 31% | ASA |
Post-stroke | Not needed if no previous stroke | ASA 30% |
Clopidogrel 43% | ||
ASA/dipyridamole 43% |
CRITERIA FOR CAROTID ENDARTERECTOMY
Carotid stenosis | Symptomatic | Asymptomatic |
|---|---|---|
≥70% | Yes (NNT 6.3) | Yes for men with stenosis ≥60% only (NNT 33) |
50–69% | Yes for men only (NNT 22) | |
<50% | No | No |
Specific Entities
DISTINGUISHING FEATURES BETWEEN UPPER MOTOR NEURON AND LOWER MOTOR NEURON LESIONS
Upper motor neuron | Lower motor neuron | |
|---|---|---|
Inspect | Atrophy after long term | Atrophy and fasciculations |
Tone | Spasticity (velocity dependent) | Flaccidity |
Strength | Upper limbs flexors > extensors, pronation > supination | Nerve root/peripheral nerve distribution |
Lower limbs extensors > flexors | ||
Reflex | Increased with clonus | Decreased |
Babinski present (upgoing toe) | Babinski absent | |
Pronator drift | Present | Absent |
APHASIA (LANGUAGE IMPAIRMENT)
testing phrases
comprehension without reply—“Touch your chin, then your nose, then your ear”
comprehension with answers—“Do you put your shoes on before your socks?”
fluency—“Describe your daily activities.”
naming—“Name this object.” (e.g. pen)
repetition—“No ifs, ands, or buts.”
DISTINGUISHING FEATURES BETWEEN DIFFERENT TYPES OF APHASIA
Wernicke | Broca | Global | Anomic | Conduction | Transcortical motor | Transcortical sensory | |
|---|---|---|---|---|---|---|---|
Fluency | Normal | – | – | Normal | Normal | – | Normal |
Comprehension | – | Normal | – | Normal | Normal | Normal | – |
Naming | – | – | – | – | – | – | – |
Repetition | – | – | – | Normal | – | Normal | Normal |
Reading | – | – | – | Normal | – | +/− | – |
Writing | Normal | – | – | Normal | – | +/− | – |
Other associated signs | Right hemiparesis /hemisensory loss | Right hemiparesis /hemisensory loss |
DYSARTHRIA (SPEECH IMPAIRMENT)
dysarthria—speech disorder resulting from disturbances in muscular control that affects respiration, articulation, phonation, resonance, or prosody
dysphonia—voice disturbance in parameters of vocal quality, pitch, or intensity
Types of dysarthria | Quality |
|---|---|
Spastic (bilateral upper motor neuron) | Harsh, strained voice |
Low pitch voice | |
Hyperkinetic (extrapyramidal [Huntington’s]) | Harsh, strained voice Low pitch voice Voice stoppages |
Hypokinetic (extrapyramidal [Parkinson’s]) | Rapid rate, monopitchLow volume |
Ataxic (cerebellar lesion) | Explosive, scanning speech |
Flaccid (LMN [myasthenia gravis]) | Breathy, nasal, low volume |
Wheezing |
PRIMITIVE REFLEXES
grasp reflex—deep pressure over palmar surface results in grasp response
suck reflex—insertion of an object into mouth results in sucking motion
root reflex—gentle stroking of cheek results in mouth turning toward that side
snout reflex—gentle pressure over the nasal philtrum results in puckering of lips
glabellar tap reflex—repeated tapping forehead produces persistent blinking
Cranial Nerve Examination
CN | Nucleus location | Skull exit | Abnormalities |
|---|---|---|---|
I | Olfactory tract | Cribriform plate | Sensory—smell (coffee, vanilla, peppermint) |
II | Thalamus | Optic canal | Sensory—visual acuity and color, visual fields, blind spot, fundoscopy Reflex—pupillary reflex (afferent) |
III | Midbrain | Superior orbital fissureb | Motor—ptosis and eye deviated downward and outward. Poor medial elevation and accommodationd Reflex—pupillary reflex (efferent) Parasympathetic—pupillary dilationd |
IV | Midbrain | Superior orbital fissureb | Motor—patient tilts head to contralateral side, vertical diplopia worst looking to one side and down |
V | Principal—Pons | V1—superior orbital fissureb V2—foramen rotundum V3—foramen ovale | Sensory—light touch, pain and temperature over V1, V2 and V3e Reflex—corneal reflex (afferent) and jaw jerk (afferent and efferent) Motor—wasting of temporal and masseter muscles, weakness of jaw movement |
Spinal—pons to spinal cord | |||
Mesencephalic—midbrain | |||
Motor—Pons | |||
VI | Pons | Superior orbital fissureb | Motor—crossed eyes, impaired lateral gaze |
VIIa | Motor, solitary, superior salivatory—Pons to midbrain | Motor—internal acoustic meatusc and stylomastoid foramen Taste—stylomastoid foramen | Sensory—numbness around the ear canal and altered taste (anterior 2/3 of tongue) Motor—difficulty raising eye brows, closing eyes, frowning, blowing out cheeks and showing teeth. Altered speech (“Pa Pa Pa”) and hyperacusis Reflex—Corneal reflex (efferent) Parasympathetic – lacrimation and saliva productionf |
VIII | Vestibular, cochlear— medulla | Internal acoustic meatusc | Sensory—whispering, Rinne’s test, Weber’s test. Dix—Hallpike maneuver (if vertigo). Check for nystagmus |
IX | Nucleus ambiguus, inferior salivatory, solitarius—medulla | Jugular foramen | Sensory—sensation of palate, taste (posterior 1/3 of tongue) Motor—uvula and palate movement. Speech (“Ka Ka Ka”), coughing, swallowing Reflex—gag reflex |
X | Nucleus ambiguus, dorsal motor vagal, solitary—medulla | Jugular foramen | Sensory—sensation of palate Motor—uvula and palate movement. Speech (“Ka Ka Ka,” hoarseness), coughing, swallowing Reflex—gag reflex |
XI | Spinal accessory—cervical cord | Jugular foramen | Motor—weakness with shrugging shoulders and rotating head against resistance |
XIIa | Medulla | Hypoglossal foramen | Motor—tongue wasting and fasciculations, tongue deviation (toward affected side). Altered speech (“La La La”) |
Specific Entities
VISUAL FIELD DEFECTS
monocular visual loss—lesion is located before optic chiasm (optic nerve, eye pathology)
bitemporal hemianopia—lesion is at the optic chiasm. The pituitary gland lies below the optic chiasm. A pituitary adenoma may compress the optic chiasm inferiorly, causing superior bitemporal quadranopsia and eventually complete bitemporal hemianopia
homonymous hemianopia—lesion is located post optic chiasm
formal visual field testing—Goldman perimeter
OCULAR FINDINGS IN HYPERTENSION AND DIABETES
hypertension—see p. 65
diabetes—see p. 381
Related Topics
Diplopia (p. 347)
Dysarthria (p. 344)
Facial Droop (p. 348)
Ptosis (p. 360)
DISTINGUISHING FEATURES BETWEEN PAPILLEDEMA, OPTIC ATROPHY, AND OPTIC NEURITIS
Papilledema | Optic atrophy | Optic neuritis | |
|---|---|---|---|
Etiology | ↑ ICP | Neuritis | Multiple sclerosis |
Tumors | Glaucoma | ||
Malignant hypertension | Congenital | ||
Symptoms | Headaches | ↓ vision | ↓ vision |
N&V, ↓ level of consciousness | ↓ color | ↓ color | |
Focal deficits | Eye pain | ||
Optic disc | Swollen optic disc | Gray–white optic disc | Swollen optic disc |
Disc margins obscured | |||
Other signs | Flame hemorrhages | ↓ acuity | ↓ acuity |
Cotton wool spots | ↓ color vision | ↓ color vision | |
↑ blind spot | ↓ pupil reflex | ↓ pupil reflex | |
↑ blind spot |
MEDULLARY SYNDROMES
Medial (Dejerine syndrome) | Lateral (Wallenberg syndrome) | |
|---|---|---|
Artery supply | Vertebral and anterior spinal arteries | Vertebral artery or posterior inferior cerebellar artery |
Structures (ipsilateral) | Hypoglossal nucleus & CN XII—tongue weakness | Trigeminal nucleus & tract—↓ facial sens Vestibular nuclei—nystagmus, vertigo, nausea, ataxia Nucleus ambiguus—dysphagia, hoarseness Nucleus solitaries—altered taste Sympathetic—Horner’s |
Motor (contralateral) | Pyramidal tract—UMN weakness) | None |
Sensory (contralateral) | Medial lemniscus—vibration, proprioception | Spinothalamic tract—↓ pain and temperature |
Cerebellum (ipsilateral) | Normal | Inferior cerebellar peduncle—ataxia |
Diplopia
Differential Diagnosis
BINOCULAR DIPLOPIA
(resolves with one eye closed, suggestive of ocular misalignment)
cranial nerves—III, IV, VI palsy, internuclear ophthalmoplegia
rectus muscles—myasthenia gravis, trauma
MONOCULAR DIPLOPIA
(persists with one eye closed, suggestive of intrinsic eye disease)
cornea—deformity, keratoconus
lens—cataract, displaced lens
retina—macular scarring
Pathophysiology
EXTRAOCULAR EYE MOVEMENTS
Muscle | Nerve | Movement |
|---|---|---|
Superior rectus | III | Elevation and intorsion |
Inferior rectus | III | Depression and extorsion |
Lateral rectus | VI | Abduction |
Medial rectus | III | Adduction |
Superior oblique | IV | Depression and intorsion |
Inferior oblique | III | Elevation and extorsion |
Clinical Features
HISTORY
—determine whether diplopia resolves with one eye closed, which direction diplopia is worse, whether separation of images occur vertically, horizontally, or obliquely, whether any head position makes diplopia better, and whether diplopia is worse at distance (typically lateral rectus palsy) or near (typically medial rectus palsy). Characterize duration, progression, limitation of function and any pain. Past medical history (head injury, stroke, infections, aneurysm, myasthenia gravis) and medications
PHYSICAL
—inspect for eye position, corneal abrasion, cataract, ptosis (CN III palsy, myasthenia gravis), eyelid retraction (thyroid ophthalmopathy), and extraocular eye movements (each eye individually, then both eyes together). Palpate for bony tenderness. Auscultate over eye for bruit of carotid cavernous fistula. Also check visual acuity, visual fields, pupil size, pupillary reflex, exophthalmos, and examine the other cranial nerves (particularly II, V, VII)
Investigations
BASIC
imaging—CT head, MR skull/orbit
SPECIAL
edrophonium or ice test—if suspect myasthenia gravis
Management
TREAT UNDERLYING CAUSE
—extraocular muscle surgery, prisms
Specific Entities
INTERNUCLEAR OPHTHALMOPLEGIA (INO)
pathophysiology—lesion in the medial longitudinal fasciculus (MLF), which connects the ipsilateral VI nucleus with the contralateral III nucleus
causes—multiple sclerosis (bilateral), brainstem infarction (unilateral), infections, malignancy, metabolic
clinical features—horizontal eye movement with weak adduction of the ipsilateral eye and abduction nystagmus of the contralateral eye
Bell’s Palsy
NEJM 2004 351:13
Causes of Facial Droop
CENTRAL
(upper motor neuron)—stroke
PERIPHERAL
(lower motor neuron)
pons—infarction, glioma, multiple sclerosis
cerebellopontine angle—acoustic or facial neuroma, meningioma, cholesteatoma, lymphoma, aneurysm, sarcoidosis
internal auditory canal proximal to or involving geniculate ganglion—Bell’s palsy, Ramsay Hunt syndrome (VZV), acoustic or facial neuroma
distal to internal auditory canal and geniculate ganglion—Bell’s palsy, temporal bone fracture, cholesteatoma, glomus tumor, middle-ear infection
stylomastoid foramen—head injury, parotid tumor
Pathophysiology
INNERVATION
—the upper facial muscles are innervated by both cerebral hemispheres, while the lower facial muscles are only innervated by the contralateral cerebral hemisphere. Thus, an upper motor neuron lesion would spare the upper face, while a lower motor neuron lesion would lead to ipsilateral upper and lower facial weakness
Clinical Features
DISTINGUISHING FEATURES BETWEEN UPPER AND LOWER MOTOR NEURON FACIAL NERVE LESIONS
Central (stroke) | Peripheral (Bell’s palsy) | |
|---|---|---|
Lesion | Contralateral cortex or corticobulbar fibers | Ipsilateral facial nerve nucleus or facial nerve |
Upper facial muscles | Furrows present | No furrows |
Can close eyes | Cannot close eyes | |
Lower facial muscles | Unable to show teeth | Unable to show teeth |
Salivation, taste, and lacrimation | Normal | Varies depending on lesion locationa |
Other findings | Hemiplegia (same side as palsy) | Hyperacusis |
Investigations
BASIC
labs—CBCD, fasting glucose
SPECIAL
imaging—MRI head (in atypical cases)
central causes workup—Lyme serology, VDRL, HIV serology, lumbar puncture
electroneurography—if persistent facial paralysis after 1 week of treatment
Diagnostic and Prognostic Issues for Bell’s Palsy
INVESTIGATIONS
—consider if other cranial nerve deficits develop, no recovery in 3–6 weeks, facial twitch or spasm precedes Bell’s palsy (suggestive of tumor)
PROGNOSIS
—71% of untreated patients recover spontaneously
Management of Bell’s Palsy
TREAT UNDERLYING CAUSE
—prednisone 1 mg/kg PO ×7 days (given within 3 days of onset). For severe facial weakness, consider valacyclovir 1 g PO TID ×7 days. Surgical decompression (only if documented 90% nerve degeneration by electroneurography)
Specific Entities
RECURRENT OR BILATERAL FACIAL PALSY
—Guillain–Barre syndrome, myasthenia gravis, lesions at skull base (lymphoma, sarcoidosis, Lyme disease)
RAMSAY HUNT SYNDROME
—reactivation of herpes zoster virus in geniculate ganglion. Polycranial neuropathy affecting CN V, IX, X. Facial palsy, ear pain, and vesicles in external auditory meatus may be present. Taste often affected, ± vertigo. Consider antiviral therapy
Multiple Sclerosis
Differential Diagnosis
INFLAMMATORY DISEASES
—Devic’s neuromyelitis (neuromyelitis optica, combination of optic neuritis, and cervical myelopathy), acute disseminated encephalomyelitis, SLE, PAN, Sjogren’s, Behcet’s disease, granulomatous angiitis, paraneoplastic encephalomyelopathies
INFECTIONS
—Lyme neuroborreliosis, neurosyphilis, HIV, HTLV-1, PML (JC virus)
GRANULOMATOUS DISEASES
—sarcoidosis, granulomatosis with polyangiitis, lymphomatoid granulomatosis
DISEASES OF MYELIN
—adult metachromatic leukodystrophy, adrenomyeloleukodystrophy
OTHERS
—vitamin B12 deficiency, Arnold–Chiari malformation, spinocerebellar disorders
Pathophysiology
MULTIPLE SCLEROSIS
—autoimmune demyelination of the central nervous system



