18 NEUROENDOCRINE SYSTEM
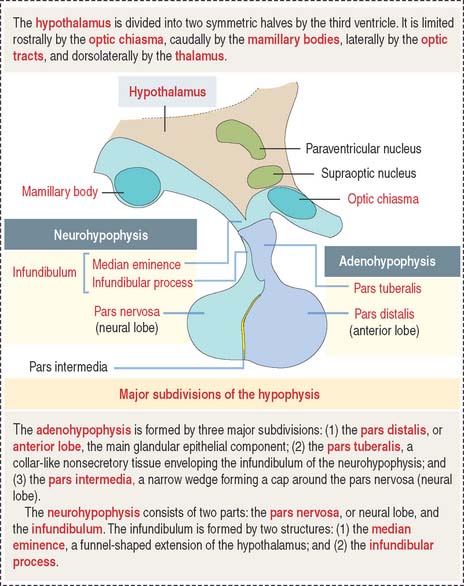
HYPOPHYSIS
The hypophysis (Greek hypo, under; physis, growth) consists of two embryo-logically distinct tissues (Figure 18-1): (1) the adenohypophysis, the glandular epithelial portion; and (2) the neurohypophysis, the neural portion.
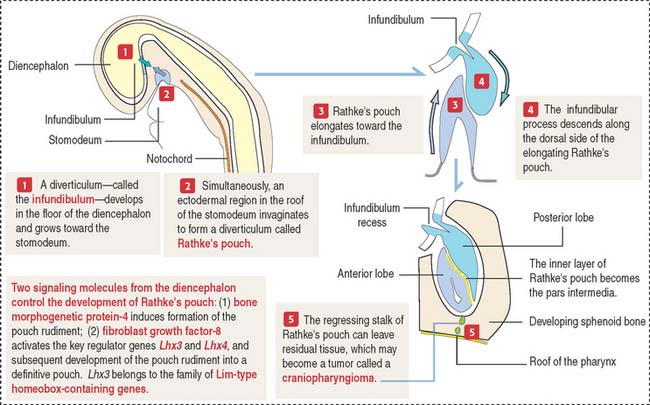
Embryologic origin of the hypophysis
The anterior hypophysis and neurohypophysis have different embryologic origins (Figure 18-2). The anterior hypophysis derives from an evagination (pouch of Rathke) of the ectodermal lining of the future oral cavity extending upward toward the developing neurohypophysis. The neurohypophysis develops from an infundibular downgrowth from the floor of the diencephalon. The connecting stem attached to the pouch of Rathke disappears. However, the connecting stem of the neurohypophysis remains as the core of the infundibular stem, or stalk.
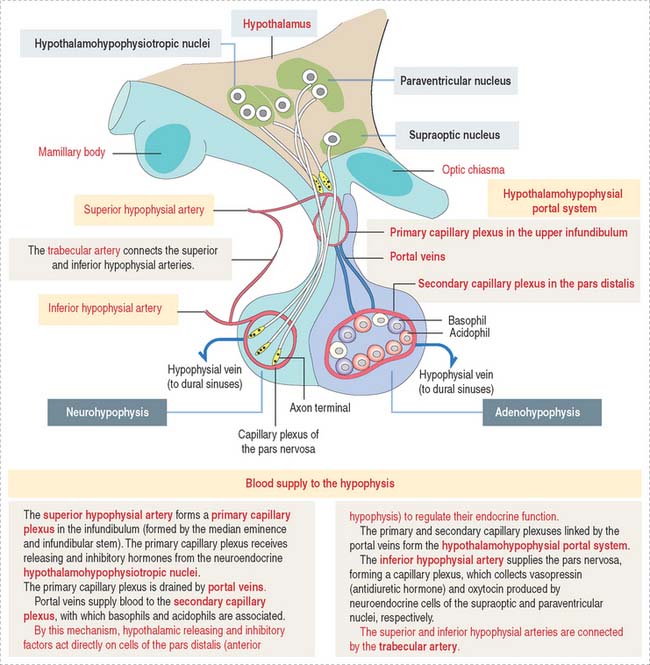
Blood supply of the hypophysis: Hypothalamohypophysial portal circulation
The superior hypophysial artery (derived from the internal carotid arteries) (Figure 18-3) enters the median eminence and upper part of the infundibular stem and forms the first sinusoidal capillary plexus (primary capillary plexus), which receives the secretion of the neuroendocrine cells grouped in the hypothalamic hypophysiotropic nuclei of the hypothalamus.
The hypothalamohypophysial portal system enables (1) the transport of hypothalamic releasing and inhibitory hormones from the primary capillary plexus to the hormone-producing epithelial cells of the anterior hypophysis; (2) the secretion of hormones from the anterior hypophysis into the secondary capillary plexus and to the general circulation; and (3) the functional integration of the hypothalamus with the anterior hypophysis, provided by the portal veins.
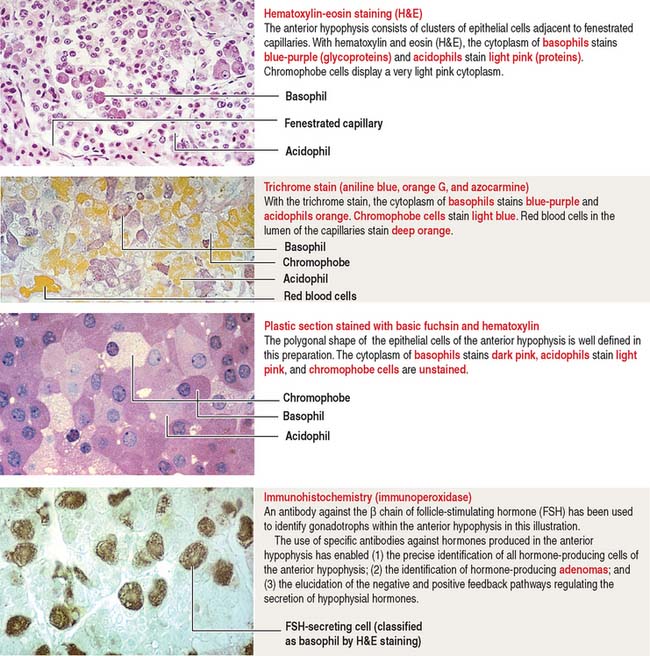
Histology of the pars distalis (anterior lobe)
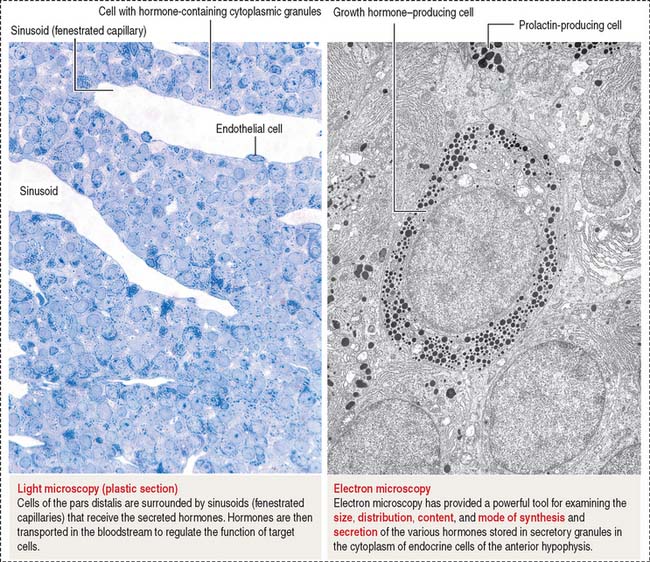
The pars distalis is formed by three components: (1) cords of epithelial cells (Figure 18-4); (2) minimal supporting connective tissue stroma; and (3) fenestrated capillaries (or sinusoids) (Figure 18-5), which are parts of the secondary capillary plexus.
There is no blood-brain barrier in the anterior hypophysis.
There are three distinct types of endocrine cell in the anterior hypophysis (see Figure 18-4): (1) acidophils (cells that stain with an acidic dye), which are prevalent at the sides of the gland; (2) basophils (cells that stain with a basic dye and are periodic acid-Schiff [PAS]-positive), which are predominant in the middle of the gland; and (3) chromophobes (cells lacking cytoplasmic staining).
Acidophils secrete two major peptide hormones: growth hormone and prolactin. Basophils secrete glycoprotein hormones: the gonadotropin folliclestimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and adrenocorticotropic hormone (ACTH), or corticotropin. Chromophobes include cells that have depleted their hormone content and lost the staining affinity typical of acidophils and basophils.
The precise identification of the endocrine cells of the anterior hypophysis is by immunohistochemistry, which demonstrates their hormone content using specific antibodies (see Figure 18-4).
Growth hormone
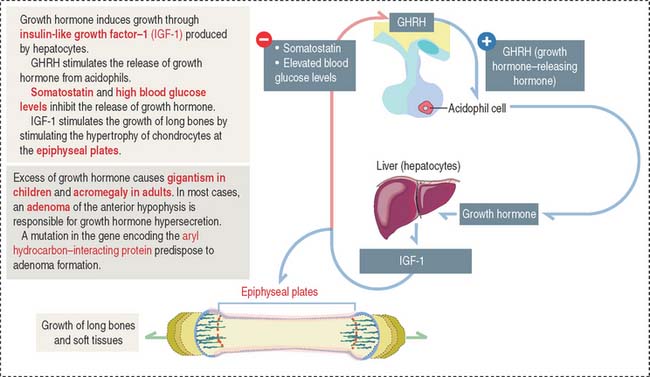
Growth hormone is a peptide of 191 amino acids in length (22 kd). It has the following characteristics (Figure 18-6): (1) Growth hormone has structural homology similar to prolactin and human placental lactogen. There is some overlap in the activity of these three hormones. (2) It is released into the blood circulation in the form of pulses throughout a 24-hour sleep-wake period, with peak secretion occurring during the first two hours of sleep. (3) Despite its name, growth hormone does not directly induce growth; rather, it acts by stimulating in hepatocytes the production of insulin-like growth factor-1 (IGF-1), also known as somatomedin C. The cell receptor for IGF-1 is similar to that for insulin (formed by dimers of two glycoproteins with integral cytoplasmic protein tyrosine kinase domains). (4) The release of growth hormone is regulated by two neuropeptides.
A stimulatory effect is caused by growth hormone–releasing hormone (GHRH), a peptide of 44 amino acids. An inhibitory effect is produced by somatostatin (a peptide of 14 amino acids) and by elevated blood glucose levels. Both GHRH and somatostatin derive from the hypothalamus. Somatostatin is also produced in the islet of Langerhans (pancreas).
Clinical significance: Gigantism (in children) and acromegaly (in adults)
Excessive secretion of growth hormone can occur in the presence of a benign tumor called an adenoma.
When the growth hormone-secreting tumor occurs during childhood and puberty at a time when the epiphyseal plates are still active, gigantism (Greek gigas, giant; extremely tall stature) is observed. If excessive growth hormone secretion occurs in the adult, when the epiphyseal plates are inactive, acromegaly (Greek akron, end or extremity; megas, large) develops. In acromegaly, the hands, feet, jaw, and soft tissues become enlarged. Long bones do not grow in length, but cartilage (nose, ears) and membranous bones (mandible and calvarium) continue to grow, leading to gross deformities.
Prolactin
The predominant action of prolactin is to stimulate the initiation and maintenance of lactation post partum (Figure 18-7). Lactation involves the following: (1) Mammogenesis, the growth and development of the mammary gland, is stimulated primarily by estrogen and progesterone in coordination with prolactin and human placental lactogen. (2) Lactogenesis, the initiation of lactation, is triggered by prolactin acting on the developed mammary gland by the actions of estrogens and progesterone. Lactation is inhibited during pregnancy by high levels of estrogen and progesterone, which decline at delivery. Either estradiol or prolactin antagonists are used clinically to stop lactation. (3) Galactopoiesis, the maintenance of milk production, requires both prolactin and oxytocin.
The effects of prolactin, placental lactogen, and steroids on the development of the lactating mammary gland are discussed in Chapter 23, Fertilization, Placentation, and Lactation.
A stimulatory effect on prolactin release is exerted by prolactin-releasing hormone (PRH) and thyrotropin-releasing hormone (TRH). Prolactin is released from acidophils in a pulsatile fashion, coinciding with and following each period of suckling. Intermittent surges of prolactin stimulate milk synthesis.
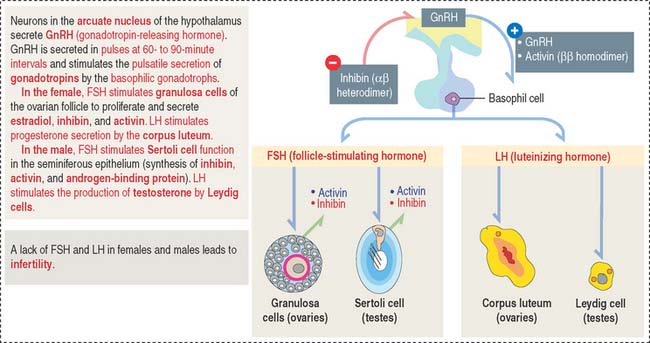
Gonadotropins: Follicle-stimulating hormone and luteinizing hormone
Gonadotrophs (gonadotropin-secreting cells) (Figure 18-8) secrete both FSH and LH. Gonadotrophs constitute about 10% of the total cell population of the anterior hypophysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree