(1)
Department of Pathology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Keywords
Thymic carcinoidAtypical carcinoidNeuroendocrine carcinomaSmall cell carcinomaLarge cell neuroendocrine carcinomaParagangliomaNeoplastic neuroendocrine proliferations of the mediastinum can originate from neuroendocrine cells that are native to the thymus, or they may arise from extra-adrenal paraganglia ectopically located in the mediastinum (i.e., paragangliomas).
Neuroendocrine carcinomas originating in the thymus are most common in middle-aged adults, with a male predilection, and can encompass the entire spectrum from well-differentiated tumors that are similar to their counterpart in other organs (i.e., carcinoid tumors) to poorly differentiated, highly malignant neoplasms (such as small cell neuroendocrine carcinomas). The term “carcinoid tumor,” which has been used in the past, is best replaced by “well-differentiated neuroendocrine carcinoma” or “low-grade neuroendocrine carcinoma,” given that the malignant potential of these lesions has now been amply established. In up to one-third of patients, these tumors can be associated with paraneoplastic syndromes, including Cushing’s syndrome, syndrome of inappropriate secretion of antidiuretic hormone, and the Eaton-Lambert syndrome. Up to one-third of cases also occur in association with the multiple endocrine neoplasia (MEN) types I and II syndromes.
The classification of neuroendocrine carcinomas in the mediastinum has remained controversial. Although the current World Health Organization (WHO) classification retains the terms “carcinoid” and “atypical carcinoid” for some of these tumors, the current trend is to use the term “well-differentiated neuroendocrine carcinoma” for “carcinoid” and to use “moderately differentiated neuroendocrine carcinoma” for “atypical carcinoid.” The new terms more accurately and realistically denote the actual biologic behavior of these tumors, whereas the WHO schema lumps typical carcinoid and atypical carcinoid under the category of well-differentiated neuroendocrine carcinoma, implying that they share a similar biologic behavior. The most recent large study (Moran and Suster, Am J Clin Pathol; 2000), however, has demonstrated that there is a stepwise progression in malignant behavior that is directly related to prognosis for these tumors as they progress from well-differentiated to moderately differentiated to poorly differentiated types. Thus, separation of well-differentiated tumors from moderately or poorly differentiated tumors is warranted.
Table 3.1 presents the criteria for histologic diagnosis of primary neuroendocrine carcinomas of the thymus. In general, increasing grade of malignancy in these tumors translates into increased cytologic atypia and loss of architectural, organotypical features of neuroendocrine differentiation. The morphologic spectrum displayed by these tumors can be quite variable, and numerous unusual morphologic variants have been described. Correct diagnosis in such instances requires awareness of these variants, which must be considered in the differential diagnosis so that an appropriate immunohistochemical workup can be initiated.
Table 3.1
Histologic classification of neuroendocrine carcinomas of the thymus
Well–differentiated neuroendocrine carcinoma |
Classic organoid growth pattern |
Minimal cytologic atypia with absence of nucleoli |
Fewer than 3 mitoses per 10 high-power fields |
No more than pinpoint, microscopic foci of necrosis |
Moderately differentiated neuroendocrine carcinoma |
Focal preservation of organoid architecture |
Moderate cytologic atypia with prominent nucleoli |
Moderate mitotic activity (3–10 mitoses per 10 high-power fields) |
Multiple comedo-like areas of necrosis |
Poorly differentiated neuroendocrine carcinoma |
Complete loss of organotypical architecture |
Marked cytologic atypia (small cell or large cell type) |
High mitotic activity (>10 mitoses per 10 high-power fields) |
Extensive “geographic” areas of necrosis |
3.1 Well-Differentiated Neuroendocrine Carcinoma (“Thymic Carcinoid”)
The growth patterns and cytologic characteristics of neuroendocrine carcinomas of the thymus (Figs. 3.1, 3.2, 3.3, 3.4, 3.5, and 3.6, Table 3.2) can be quite varied.
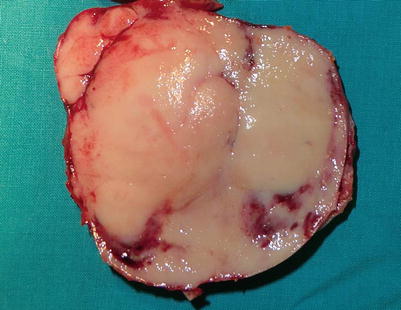
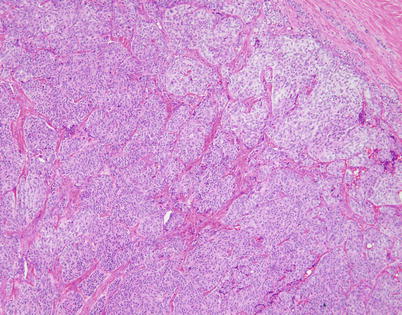
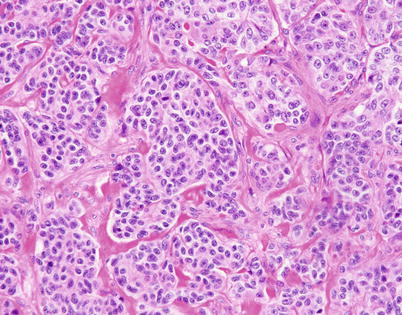
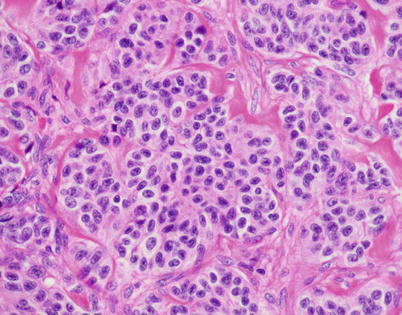
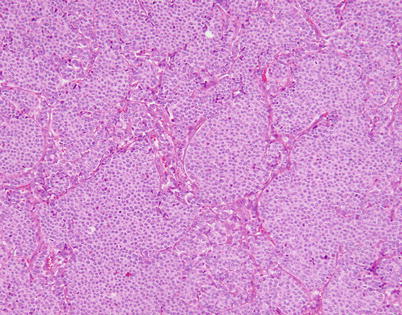
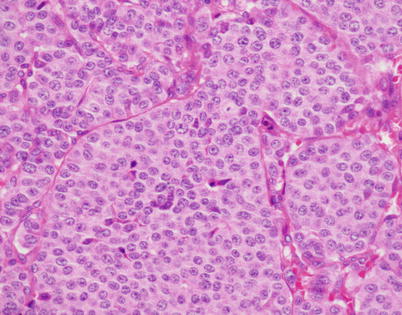

Fig. 3.1
Gross appearance of well-differentiated neuroendocrine carcinoma of the thymus (thymic carcinoid) shows a well-circumscribed, fleshy, tan-white mass with foci of hemorrhage and a vaguely lobulated cut surface

Fig. 3.2
Scanning magnification of well-differentiated neuroendocrine carcinoma of the thymus (thymic carcinoid) shows well-developed “organoid” (neuroendocrine) architecture, with discrete nests of tumor cells separated by fibrovascular stroma

Fig. 3.3
Higher magnification of well-differentiated neuroendocrine carcinoma of the thymus with “organoid” (neuroendocrine) growth pattern shows well-defined small nests of cells containing a monotonous, round cell population with abundant cytoplasm and small, round to oval nuclei with stippled (“salt-and-pepper”) chromatin pattern

Fig. 3.4
High power of well-differentiated neuroendocrine carcinoma of the thymus shows round cells with round to oval nuclei showing a stippled chromatin pattern with an ample rim of eosinophilic cytoplasm

Fig. 3.5
Another example of the organoid, nested (“tzellballen”) growth pattern in thymic neuroendocrine carcinoma shows larger nests of monotonous tumor cells separated by thin and compressed fibrovascular septa

Fig. 3.6
Higher magnification of Fig. 3.5, showing well-differentiated neuroendocrine carcinoma of the thymus with a monotonous population of small, round cells surrounded by abundant eosinophilic cytoplasm. A few isolated tumor cells show a small nucleolus, but there is no evidence of necrosis, nuclear pleomorphism, or mitotic activity
Table 3.2
Growth patterns and cytologic variants of thymic neuroendocrine carcinomas
Organoid (nested, “tzellballen,” insular) |
Trabecular |
Serpiginous/ribbon-like |
Cribriform |
Pseudoglandular |
Microacinar |
Diffuse |
Sclerosing/desmoplastic |
Oncocytic |
Clear cell |
Pigmented |
Spindle cell |
Medullary carcinoma-like |
3.2 Moderately Differentiated Neuroendocrine Carcinoma
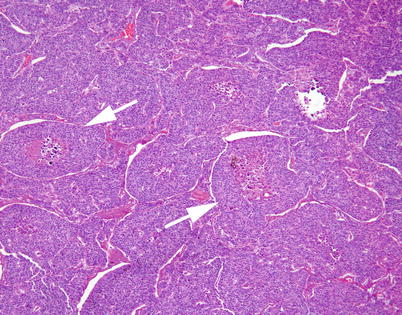
Fig. 3.7
Moderately differentiated neuroendocrine carcinoma of the thymus with organoid (nested) pattern of growth. Despite the preservation of the nested pattern, a few of the “nests” are already showing evidence of central, comedo-like necrosis (arrows)
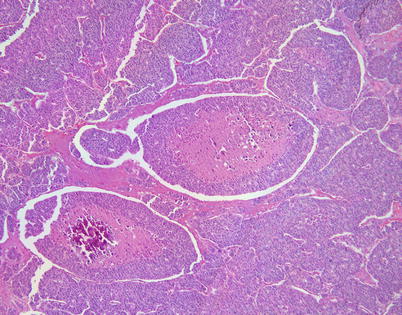
Fig. 3.8
Higher magnification of moderately differentiated neuroendocrine thymic carcinoma showing prominent central areas of comedonecrosis. The “balls” of tumor cells containing the areas of necrosis show a prominent “retraction” artifact from the surrounding tumor cells. This is a prominent and distinctive feature seen in these tumors in a mediastinal location
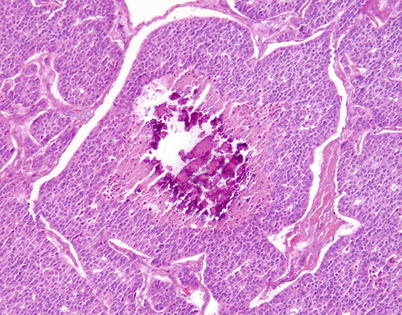
Fig. 3.9
Another example of moderately differentiated neuroendocrine carcinoma of the thymus with a focus of comedonecrosis that is undergoing dystrophic calcification. These microscopic foci of calcification are responsible for a “gritty” sensation when cutting the gross specimen
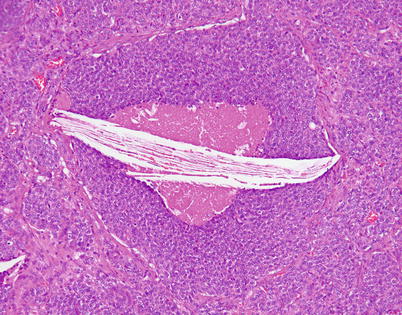
Fig. 3.10
In addition to foci of dystrophic calcification, the foci of comedonecrosis within the islands of comedonecrosis in moderately differentiated neuroendocrine carcinoma of the thymus can also contain prominent cholesterol-cleft granulomas
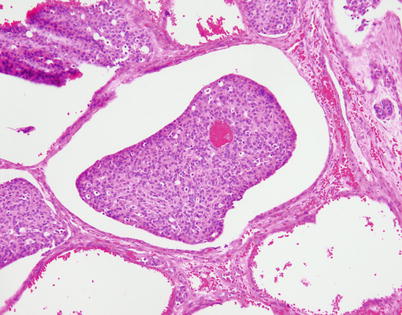
Fig. 3.11
The “retraction” artifact seen in neuroendocrine carcinomas of the thymus can sometimes be quite striking, to the extent of simulating lymphovascular invasion
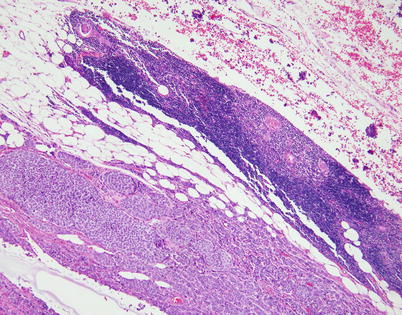
Fig. 3.12
Most neuroendocrine carcinomas of the thymus are invasive at the time of initial diagnosis and infiltrate into the surrounding fat. Residual involuting thymic rests commonly can be found at the periphery of the lesion (top right)
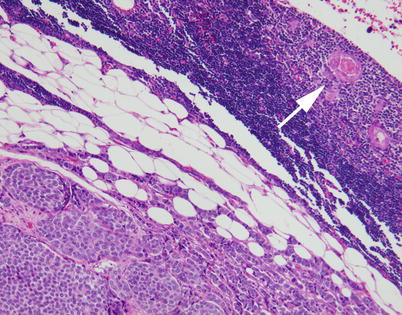
Fig. 3.13
Higher magnification from the invasive border of a thymic neuroendocrine carcinoma, showing a compressed rim of involuting thymic tissue. Notice the Hassall’s corpuscle (arrow)
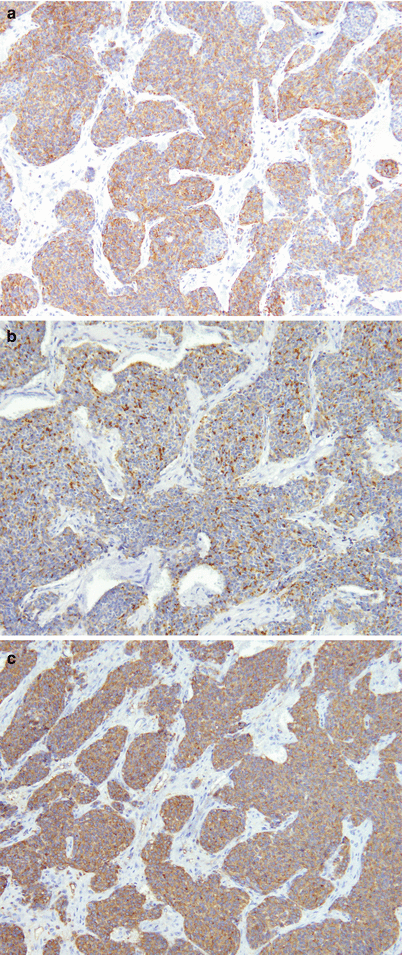
Fig. 3.14
Immunohistochemistry is very helpful for the diagnosis of neuroendocrine carcinoma of the thymus. Most cases stain very strongly with keratin antibodies, including cytokeratin AE1/AE3, broad-spectrum keratin, and low-molecular-weight cytokeratins. The tumors also will invariably express markers associated with neuroendocrine cells, including chromogranin-A, synaptophysin, and CD56. The tumor cells are negative for calcitonin, polyclonal CEA (pCEA), p63, and other differentiation markers. (a) An example of pan-cytokeratin in thymic neuroendocrine carcinoma. (b) A positive reaction for chromogranin. (c) Positive synaptophysin staining
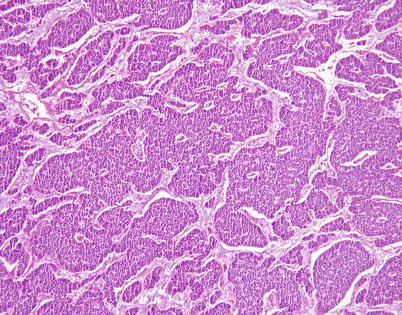
Fig. 3.15
Another growth pattern commonly observed in well-differentiated and moderately differentiated neuroendocrine carcinoma of the thymus is characterized by broad, irregular islands of monotonous tumor cells that adopt a rather serpiginous, interlocking configuration separated by bands of connective tissue. A hint of peripheral palisading of nuclei is detected at scanning magnification
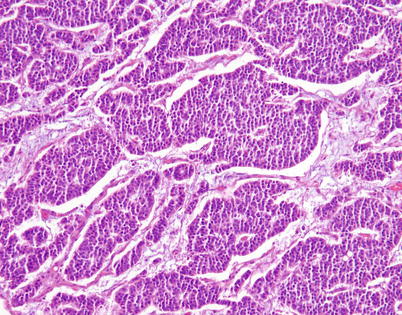
Fig. 3.16
Higher magnification from Fig. 3.15 shows irregular, serpiginous islands of monotonous tumor cells in well-differentiated neuroendocrine carcinoma of the thymus
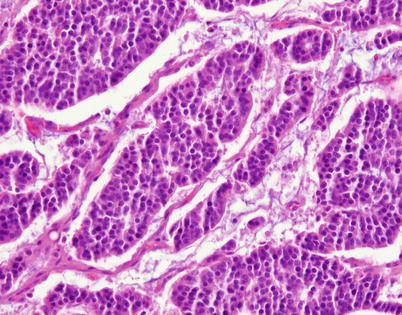
Fig. 3.17
Higher magnification in the same case shows islands of small, monotonous tumor cells devoid of significant pleomorphism or mitotic activity, with small, round central nuclei surrounded by a scant rim of eosinophilic cytoplasm
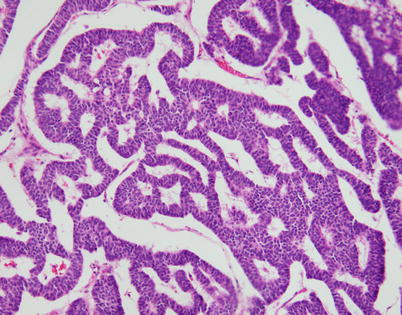
Fig. 3.18
Another common growth pattern seen in neuroendocrine carcinomas of the thymus is characterized by complex, interlacing trabeculae of tumor cells that impart a ribbon-like appearance on scanning magnification
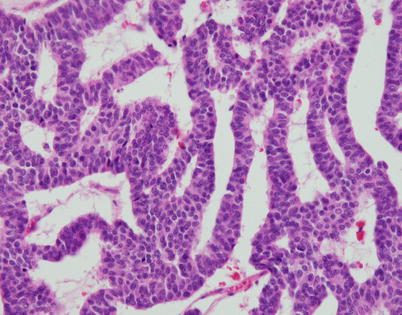
Fig. 3.19
Higher magnification of the ribbon-like pattern of growth in neuroendocrine carcinomas of the thymus shows thin cords of tumor cells composed of two to three layers of cells circumscribing empty spaces
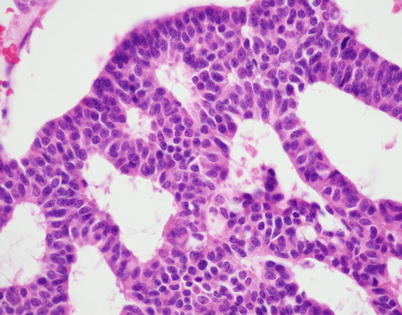
Fig. 3.20
Higher magnification of neuroendocrine thymic carcinoma with a trabecular pattern shows a monotonous population of small tumor cells with uniform, round to oval nuclei with a speckled (“salt-and-pepper”) chromatin pattern surrounded by a scant rim of eosinophilic cytoplasm. Notice the lack of mitotic activity and nuclear pleomorphism
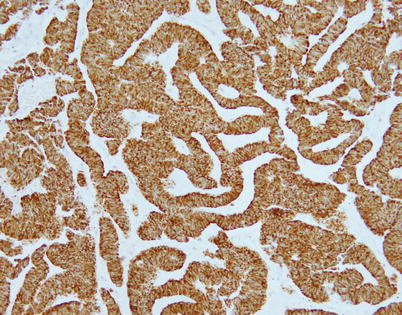
Fig. 3.21
Immunohistochemical staining of trabecular neuroendocrine carcinoma of the thymus shows positivity of the tumor cells for cytokeratin AE1/AE3
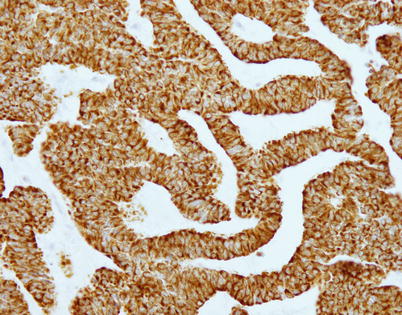
Fig. 3.22
Trabecular neuroendocrine carcinoma of the thymus also shows strong positive reaction of the tumor cells to chromogranin
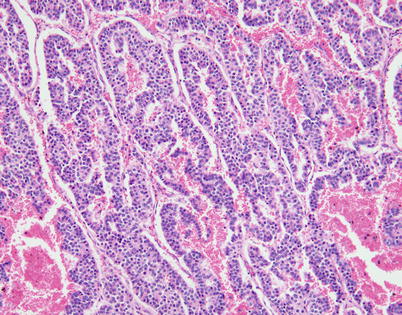
Fig. 3.23
Another variation of the trabecular/serpiginous growth pattern seen in neuroendocrine carcinoma of the thymus is characterized by anastomosing cords of tumor cells separated by prominently vascularized stroma
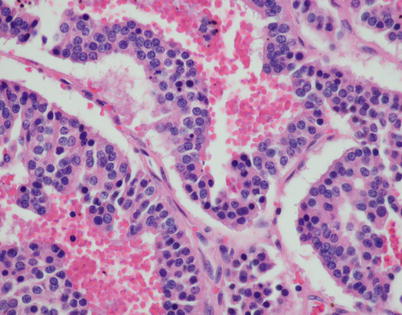
Fig. 3.24
Higher magnification from Fig. 3.23 shows the characteristic “salt-and-pepper” chromatin pattern in the monotonous cell population composing the cords of tumor cells
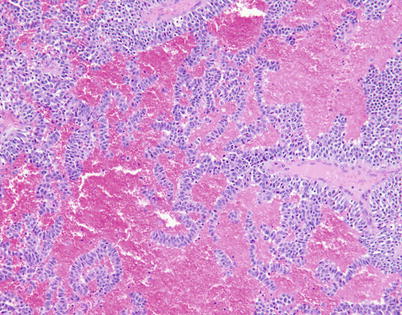
Fig. 3.25
Trabecular pattern of growth with prominent vascular stroma in moderately differentiated neuroendocrine carcinoma of the thymus shows thin ribbons of tumor cells with abundant extravasated red blood cells lying in the surrounding stroma
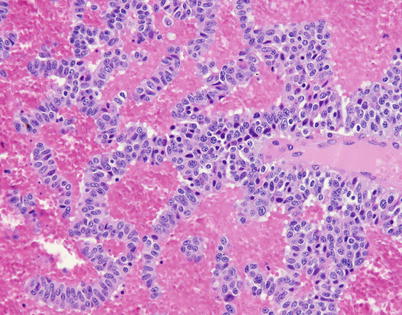
Fig. 3.26
Higher magnification from Fig. 3.25 shows cords of tumor cells showing some variation in size with mild nuclear pleomorphism. Some of the cells contain prominent nucleoli, and there are many scattered apoptotic tumor cells
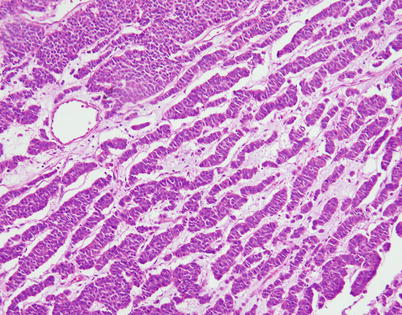
Fig. 3.27
Another example of the ribbon-like, trabecular growth pattern in moderately differentiated neuroendocrine carcinoma of the thymus shows parallel cords of tumor cells separated by loose connective tissue stroma
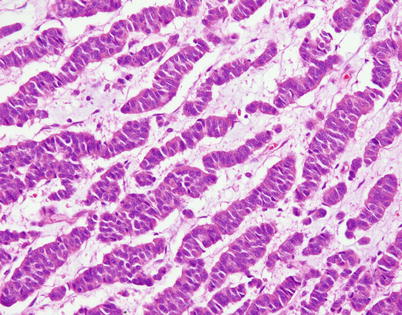
Fig. 3.28
Higher magnification from Fig. 3.27 shows cords of tumor cells with round to oval nuclei and mild nuclear pleomorphism
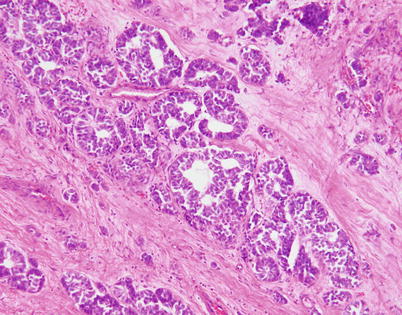
Fig. 3.29
Moderately differentiated neuroendocrine carcinoma of the thymus with a pseudoglandular growth pattern. Tumors with these features can pose a serious pitfall for diagnosis, being mistaken for metastatic carcinoma or primary thymic adenocarcinoma
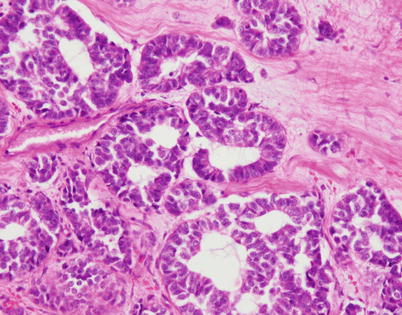
Fig. 3.30
Higher magnification of moderately differentiated neuroendocrine carcinoma of the thymus shows structures resembling glands with a cribriform appearance and well-formed, empty lumens. Immunohistochemical stains for chromogranin and synaptophysin demonstrated the neuroendocrine nature of the tumor cells; these areas merged with more conventional neuroendocrine-appearing components in other sections of this tumor
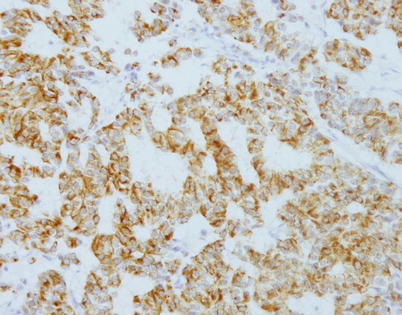
Fig. 3.31
Immunohistochemical staining with antibodies against chromogranin-A in pseudoglandular neuroendocrine carcinoma of the thymus shows positive cytoplasmic staining of the tumor cells outlining the pseudoglandular spaces
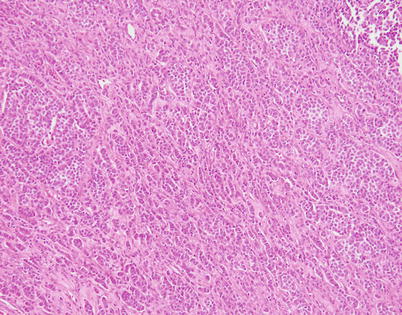
Fig. 3.32
Another example of moderately differentiated neuroendocrine carcinoma of the thymus that can be confused for a metastatic carcinoma is characterized by a striking, single-file pattern of growth that closely simulates metastatic breast carcinoma
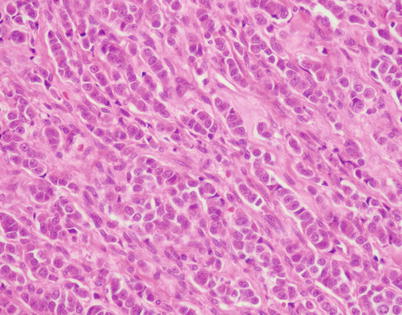
Fig. 3.33
Higher magnification of the case in Fig. 3.32 shows thin cords of tumor cells in a parallel, single-file arrangement within connective tissue, simulating an invasive carcinoma of the breast. Immunohistochemical stains are required to distinguish this lesion from a metastasis from breast carcinoma
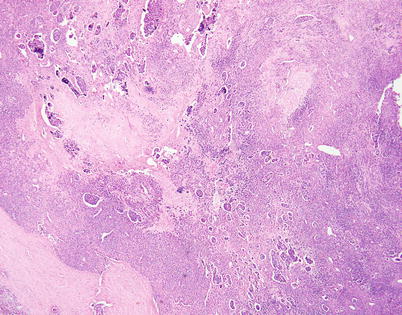
Fig. 3.34




Stromal changes can increase the difficulty of properly recognizing neuroendocrine carcinomas of the thymus. Massive stromal sclerosis can lead to the formation of sheets of individually scattered small tumor cells entrapped within the hyalinized stroma, giving an impression of a mesenchymal neoplasm. Small islands of compact tumor cells seen scattered in the background show the distinctive “retraction” artifact in this example of moderately differentiated neuroendocrine carcinoma of the thymus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


