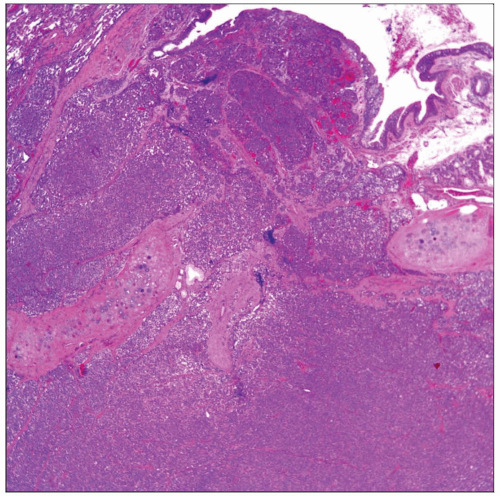
Neuroendocrine Carcinoma (Including Small Cell Carcinoma)
Key Facts
Terminology
Spectrum of neoplasms ranging from low- to high-grade malignancy
Clinical Issues
Paraneoplastic syndromes
Macroscopic Features
Endobronchial or intraparenchymal tumor
0.5 to > 10 cm in diameter
Microscopic Pathology
Neuroendocrine pattern, mitotic activity, necrosis
Low-grade tumors: < 2 mitotic figures per 10 HPF; absence of necrosis
Intermediate-grade tumor: 3-10 mitotic figures per 10 HPF; comedo-like necrosis
High-grade tumors: > 10 mitotic figures per 10 HPF; necrosis is present
Large cell neuroendocrine carcinoma requires neuroendocrine pattern and positive staining with neuroendocrine markers (chromogranin-A, synaptophysin, CD56)
In small cell carcinoma, mitotic count of > 10 per 10 HPF applies only to resected specimens, not biopsy material
Ancillary Tests
Chromogranin-A
Synaptophysin
CD56
Diagnostic Checklist
Mitotic rate
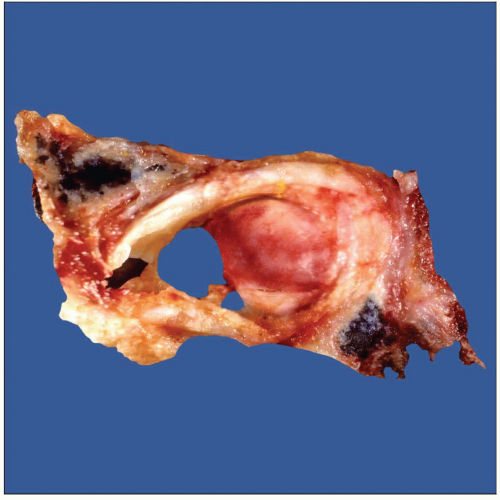 Gross photograph of neuroendocrine carcinoma shows an endobronchial tumor obstructing approximately 50% of the bronchial lumen. |
TERMINOLOGY
Abbreviations
Typical carcinoid (TC), atypical carcinoid (AT), small cell neuroendocrine carcinoma (SCNEC), large cell neuroendocrine carcinoma (LCNEC)
Synonyms
Carcinoid tumor, atypical carcinoid, small cell carcinoma
Definitions
Spectrum of neoplasms ranging from low- to high-grade malignancy showing neuroendocrine differentiation
ETIOLOGY/PATHOGENESIS
Etiology
Tumor is thought to be derived from Kulchitsky cells
CLINICAL ISSUES
Presentation
Cough
Incidental finding
Hemoptysis
Paraneoplastic syndromes
Chest pain
Weight loss
Dyspnea
Treatment
Surgical approaches
Low- and intermediate-grade tumor
Adjuvant therapy
High-grade tumors
Prognosis
Low-grade neoplasms
Survival rate at 5 years: > 75%
Intermediate-grade neoplasms
Survival rate at 5 years: ˜ 50%
High-grade neoplasms
Survival rate at 5 years: May be < 5%
MACROSCOPIC FEATURES
General Features
Endobronchial or intraparenchymal tumor
High-grade tumor may show extensive areas of necrosis
Size
0.5 to > 10 cm in diameter
MICROSCOPIC PATHOLOGY
Histologic Features
Low-grade tumors
< 3 Mitotic figures per 10 HPF
Necrosis is absent
Intermediate-grade tumors
3-10 mitotic figures per 10 HPF
Comedo-like necrosis
High-grade tumors
> 10 Mitotic figures per 10 HPF
Necrosis is present
Large cell neuroendocrine carcinoma requires neuroendocrine pattern and positive staining with neuroendocrine markers (chromogranin-A, synaptophysin, CD56)
Cells with prominent nucleoli
Neuroendocrine markers must be positive
Electron microscopic studies show neurosecretory granules
Comedo-like necrosis
Small cell carcinoma
Miotic figures > 10 per 10 HPF applies only to resected specimens
Neuroendocrine markers are not required for diagnosis
Predominant Pattern/Injury Type
Nesting
Diffuse
Mucinous
Glandular
Predominant Cell/Compartment Type
Oncocytic
Spindle
Melanocytic
Epithelial, neuroendocrine
Clear
DIFFERENTIAL DIAGNOSIS
Low-Grade Neuroendocrine Carcinoma
< 3 mitotic figures and absence of necrosis
Well-organized growth pattern
Intermediate-Grade Neuroendocrine Carcinoma
Mitotic activity from 3-9 per 10 HPF and necrosis
Often a combination of well-organized nested pattern and diffuse pattern of growth
High-Grade Neuroendocrine Carcinoma
> 10 mitotic figures per 10 HPF, necrosis &/or hemorrhage (in resected specimens)
Positive neuroendocrine markers (synaptophysin, chromogranin-B, &/or CD56) in cases of large cell neuroendocrine carcinoma
In cases of small cell carcinoma, neuroendocrine markers may be negative
Carcinoid Tumorlet
These tumors are usually < 5 mm in diameter
Tumorlets and carcinoid tumors share same immunophenotype
Metastatic Neuroendocrine Carcinoma of Extrathoracic Origin
Clinical history of previous tumor is of utmost importance
Immunohistochemical study for TTF-1 may be helpful
Pulmonary Paraganglioma
Paragangliomas and neuroendocrine tumors show positive staining for neuroendocrine markers
Paragangliomas are usually negative for keratin
Paragangliomas generally do not show mitotic activity
Paragangliomas usually show cells with macronuclei
Large Cell Carcinoma
Large cell neuroendocrine carcinoma must show neuroendocrine pattern and positive neuroendocrine markers
Large Cell Carcinoma with Neuroendocrine Differentiation
Histology is that of conventional non-small cell carcinoma with positive neuroendocrine markers
Large Cell Carcinoma with Neuroendocrine Pattern
Tumors show neuroendocrine histologic pattern but negative staining for neuroendocrine markers
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
Mitotic rate
GRADING
Low-Grade Neuroendocrine Carcinoma (Carcinoid Tumor)
Tumors with < 3 mitoses per 10 HPF and no necrosis
Intermediate-Grade Neuroendocrine Carcinoma (Atypical Carcinoid)
Tumors with ≥ 3 but > 10 per 10 HPF and necrosis
High-Grade Neuroendocrine Carcinoma
Small cell carcinoma
Large cell neuroendocrine carcinoma
For large cell neuroendocrine carcinoma, neuroendocrine markers must be positive
SELECTED REFERENCES
1. Moran CA et al: Neuroendocrine carcinomas of the lung: a critical analysis. Am J Clin Pathol. 131(2):206-21, 2009
2. Segawa Y et al: Immunohistochemical detection of neuroendocrine differentiation in non-small-cell lung cancer and its clinical implications. J Cancer Res Clin Oncol. 135(8):1055-9, 2009
3. Di Fabio R et al: Paraneoplastic neuromuscular disease in lung large cell neuroendocrine carcinoma. Can J Neurol Sci. 35(4):516-8, 2008
4. Dörffel Y et al: Neuroendocrine tumors: characterization with contrast-enhanced ultrasonography. Ultraschall Med. 29(5):506-14, 2008
5. García-Yuste M et al: Neuroendocrine tumors of the lung. Curr Opin Oncol. 20(2):148-54, 2008
6. Gustafsson BI et al: Bronchopulmonary neuroendocrine tumors. Cancer. 113(1):5-21, 2008
7. Namwongprom S et al: Correlation of chromogranin A levels and somatostatin receptor scintigraphy findings in the evaluation of metastases in carcinoid tumors. Ann Nucl Med. 22(4):237-43, 2008
8. Pinchot SN et al: Carcinoid tumors. Oncologist. 13(12):1255-69, 2008
9. Sica G et al: Immunohistochemical expression of estrogen and progesterone receptors in primary pulmonary neuroendocrine tumors. Arch Pathol Lab Med. 132(12):1889-95, 2008
10. Thomas R et al: Clinico-pathologic study of pulmonary carcinoid tumours—a retrospective analysis and review of literature. Respir Med. 102(11):1611-4, 2008
11. Tschernatsch M et al: Paraneoplastic neurological syndromes in patients with carcinoid. Eur J Neurol. 15(12):1390-4, 2008
12. Yao JC et al: One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 26(18):3063-72, 2008
13. Moran CA et al: Neuroendocrine carcinomas (carcinoid, atypical carcinoid, small cell carcinoma, and large cell neuroendocrine carcinoma): current concepts. Hematol Oncol Clin North Am. 21(3):395-407; vii, 2007
14. Cerilli LA et al: Neuroendocrine neoplasms of the lung. Am J Clin Pathol. 116 Suppl:S65-96, 2001
15. Travis WD et al: Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 22(8):934-44, 1998
16. Dresler CM et al: Clinical-pathologic analysis of 40 patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg. 63(1):180-5, 1997
17. Arrigoni MG et al: Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg. 64(3):413-21, 1972
Tables
Immunohistochemistry | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree