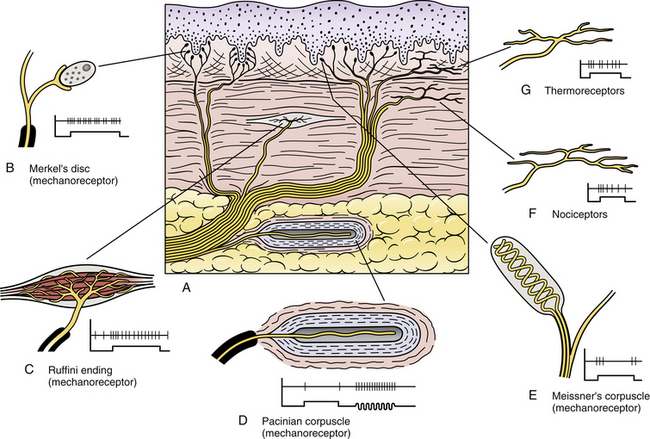
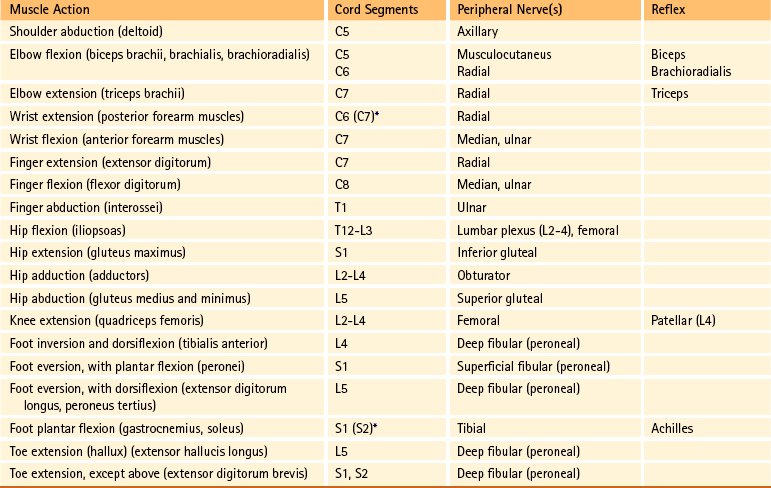
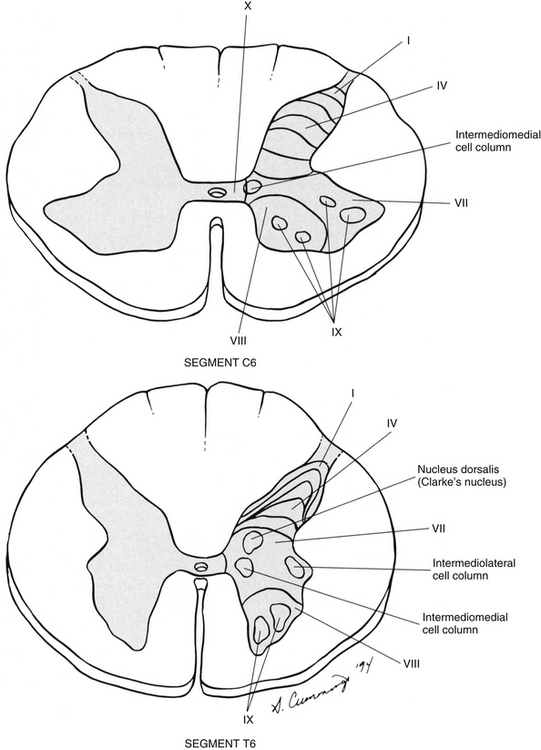
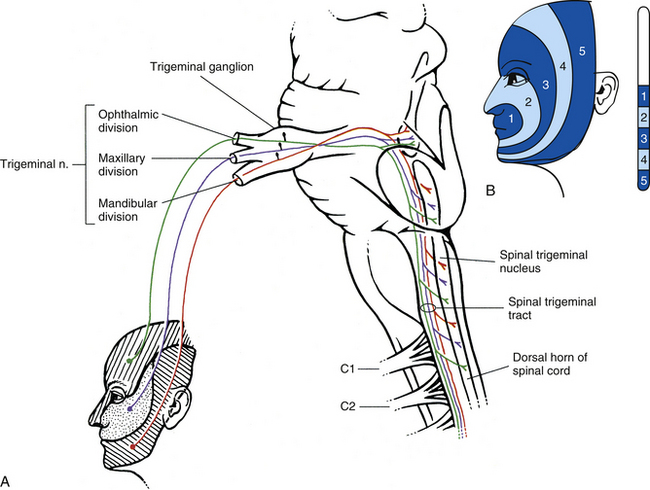
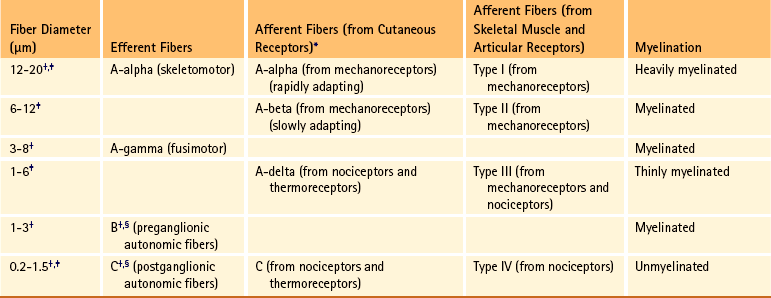
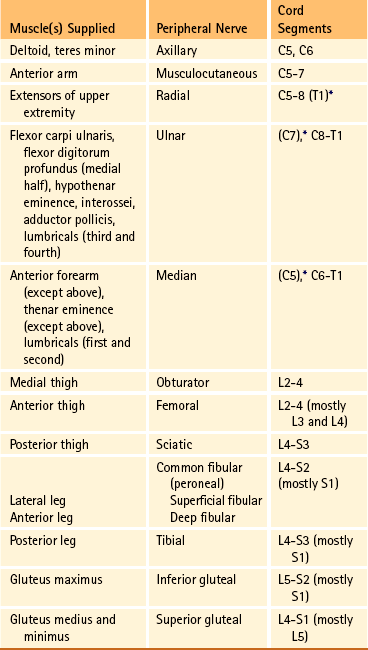
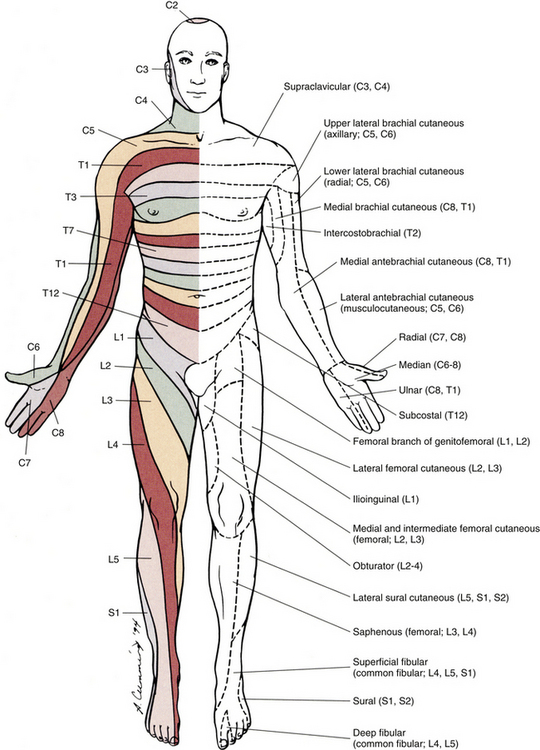
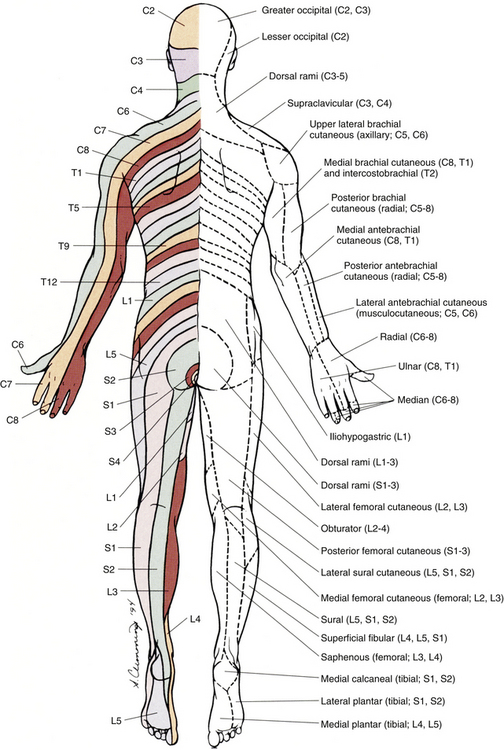
Chapter 9 The vertebral column and its adjacent musculature are discussed in detail in the previous chapters. Because of the intimate anatomic and functional relationship between the vertebral column and spinal cord (which is protected by the vertebral column), knowledge of both is equally important. Chapter 3 describes the meninges, external surface, and vasculature of the spinal cord. In addition, it provides a cursory description of the cord’s internal organization. The purpose of this chapter is to elaborate on this internal organization by discussing the neurons, which form the circuitry of the spinal cord, and the ascending and descending tracts, which provide a connection among the spinal cord, the peripheral nerves, and the higher centers of the central nervous system. This information forms the basis of important neuroanatomic concepts that are necessary for an understanding of clinical neuroscience. The knowledge of these concepts is imperative for diagnosing pathologic conditions of the cord, some of which may be caused by vertebral column dysfunction. Examples demonstrating the application of these neuroanatomic principles to pathologic conditions are presented at the end of the chapter. The sensory neurons of the peripheral nervous system (PNS) are pseudounipolar neurons. Their cell bodies are located in the dorsal root ganglia. The peripheral process, which may be myelinated or unmyelinated, is the part of the fiber continuous with the receptor. It is the sensory component of a peripheral nerve. The other part of the fiber, the central process, enters the CNS. A bundle of central processes forms a dorsal root. The peripheral processes are classified according to their conduction velocity, and conduction velocity is related to the axon diameter. Fibers with large diameters conduct the fastest. Based on the relationship between velocity and diameter, cutaneous fibers are classified alphabetically as Aβ (beta), Aδ (delta), and C fibers. Similarly, afferents from muscle tissue usually are classified numerically from heavily myelinated to unmyelinated as I, II, III, and IV. Type I also has subgroups of Ia and Ib. Afferents from visceral interoceptors often are classified as Aδand C fibers. Motor (efferent) fibers also are classified according to the alphabetic listing. Large somatic motor neurons correspond to the Aα(alpha) and Aγ(gamma) group, and autonomic efferent fibers correspond to the B and C groups. Table 9-1 summarizes the classifications of the afferent and efferent fibers. Table 9-1 Summary of the Classification of Peripheral Fibers ∗Afferent fibers from visceral receptors are classified as A-delta and C fibers. †From Kiernan JA. (2009). The human nervous system (7th ed.). Philadelphia: JB Lippincott. ‡From Gardner EP, Martin JH, & Jessell TM. (2000). The bodily senses. In ER Kandel, JH Schwartz, & TM Jessell (Eds.). Principles of neural science (4th ed.). New York: McGraw-Hill. §From Standring et al. (2008). Gray’s anatomy (38th ed.). Edinburgh: Churchill Livingstone. Peripheral receptors can be classified by their morphology, location, and the type of stimulus to which they respond. Morphologically, receptors may be encapsulated by connective tissue and nonneural cells, or they may simply be nonencapsulated, bare arborizing endings. Receptors classified by their location of distribution are called exteroceptors, proprioceptors, or interoceptors. Exteroceptors are superficial and located in the skin. Modalities such as nociception (pain), temperature, and touch (and the submodalities of pressure and vibration) are conveyed by these receptors. Proprioceptors are located in the muscles, tendons, and joints of the body and provide information concerning limb position, while the limbs are either stationary (static) or moving (dynamic or kinesthetic) (Pearson & Gordon, 2000a; Nolte, 2002; Standring et al. 2008; Kiernan, 2009). Interoceptors are located in the viscera, glands, and vessels and convey poorly localized information from such systems as the digestive and urinary systems. Examples of the types of information conveyed by interoceptors include distention or fullness and ischemic pain. Cutaneous receptors (exteroceptors) include mechanoreceptors, thermoreceptors, and nociceptors and subserve such modalities as touch, pressure, vibration, temperature, and nociception (pain) (Fig. 9-1). Mechanoreceptors include the nonencapsulated Merkel’s discs, nonencapsulated endings surrounding hair follicles (peritrichial), and encapsulated endings such as Ruffini endings, pacinian corpuscles, and Meissner’s corpuscles. The fibers supplying these receptors are primarily A-beta. Thermoreceptors are nonencapsulated, free nerve endings that occupy areas approximately 1 mm in diameter. Cold thermoreceptors respond in the range of 5° C (41° F) to 40° C (104° F) relative to the normal skin temperature of 34° C (93.2° F) and fire most frequently at 25° C (77° F). Warm thermoreceptors are stimulated in the temperature range 29° C (84.2° F) to 45° C (113° F) and are most active at 45° C (113° F) (Gardner, Martin, & Jessell, 2000). Cold receptors are supplied by A-delta or C fibers, but warm receptors are supplied by C fibers alone. An understanding of nociceptors is helpful in the comprehension of pain of spinal origin. Nociceptors are free nerve endings, and they respond to stimuli that may threaten or actually damage adjacent tissue cells. The damage to cells causes the release of chemical mediators that either may sensitize (e.g., prostaglandins, leukotrienes, substance P) previously unresponsive free nerve endings by lowering their threshold for activation or may activate (e.g., histamine, bradykinin, potassium, serotonin) the free nerve endings. In some instances, activated free nerve endings release substance P and calcitonin gene–related peptide into the surrounding area, causing vasodilation, extravasation of fluid, and release of histamine from tissue cells. These changes in turn lead to lowering of the threshold for activation of more nociceptors. The inflammatory process that results from the activity of these nociceptors is termed neurogenic inflammation (Basbaum & Jessell, 2000). Three types of nociceptors appear to exist: (a) mechanical, which are stimulated by mechanical damage such as by a sharp object; (b) thermal, which are stimulated by temperatures higher than 45° C (113° F) or lower than 20° C (68° F); and (c) polymodal, which respond to damaging mechanical, thermal, or chemical stimuli. Mechanical and thermal nociceptors send their information via A-delta fibers, whereas polymodal receptors use C fibers. The three types of nociceptors often are activated simultaneously and thus work together. For example, if a person stubs a toe, initially there is sharp (fast) pain resulting from stimulating mechanical nociceptors followed by an achy prolonged pain resulting from stimulating polymodal nociceptors that use slower C fibers. The cutaneous fibers of these receptors form overlapping horizontal plexuses in the dermis and subcutaneous layers of the skin. The density and variety of receptors vary in different regions. For example, in hairy skin the peritrichial endings are most common, but Merkel’s discs and free nerve endings are also present. In glabrous (hairless) skin, free nerve endings are present, as are Merkel’s discs and Meissner’s corpuscles. The latter two receptors have small receptive fields and help to discriminate the spatial relationship of stimuli. This ability to discriminate is well developed on the fingertips. In fact, Meissner’s corpuscles have been located only in primate animals (Kiernan, 2009). The subcutaneous tissues of both types of skin are provided with pacinian corpuscles and Ruffini endings, both of which have large receptive fields and therefore are less discriminatory (Gardner, Martin, & Jessell, 2000). The majority of the receptors located in muscles, tendons, and joints are involved with the sense of proprioception. The receptors are classified as proprioceptors based on their location. They are classified as mechanoreceptors based on the type of stimulus to which they respond. These receptors convey proprioception, the term used to describe the sensory information that contributes to the sense of movement and position of one’s own limbs and body without visual input. The mechanoreceptors involved with providing proprioception (excluding the vestibular system of the inner ear) are the joint receptors, neuromuscular spindles, and Golgi tendon organs (neurotendinous spindles). They function in the coordination and control of movements and the maintenance of upright posture by monitoring both the stationary position and the movement (kinesthesia) of body parts, and then relaying that information into the CNS. This information, often called joint position sense, may be perceived consciously. In addition, cutaneous mechanoreceptors also may function in the overall assessment of proprioception. For example, they may aid other proprioceptors in the task of discriminating different thicknesses of objects held between the thumb and finger and in the manipulation of objects of different shapes and sizes (McCloskey, 1994; Gardner, Martin, & Jessell, 2000). Joint receptors are located in the superficial and deep layers of the joint capsules and in the ligaments. Four types of receptors exist, classified as I, II, III, and IV. The first three types are encapsulated mechanoreceptors that have a proprioceptive function. The fourth type consists of unmyelinated free nerve endings. The receptors classified as I, II, or III provide information about such activities as the direction, velocity, and initiation of joint movements. They do this by responding to tension applied to the connective tissue surrounding them. The group IV free nerve endings, which mediate nociception and normally are silent, respond to potentially injurious mechanical or inflammatory processes (Wyke, 1985). Neuromuscular spindles are complex encapsulated proprioceptors that monitor muscle fiber length (see Fig. 9-24). They are located within a skeletal muscle close to the tendon and are surrounded by muscle fibers. They provide the sensory arc of the stretch, or myotatic, reflex. Each spindle consists of a connective tissue capsule that is fixed at each end to adjacent muscle fibers. The capsule encloses specialized muscle fibers called intrafusal fibers, which are either nuclear bag fibers or nuclear chain fibers. The total and individual numbers of nuclear bag and chain fibers vary among spindles. The innervation of the nuclear bag and chain fibers of the spindle is provided by group Ia and II sensory fibers. In addition, the spindle is a unique peripheral receptor in that it also has a motor innervation furnished by gamma motor neurons. The motor innervation of the intrafusal fibers allows the spindle to remain sensitive to muscle fiber length during muscle contraction. The section Spinal Motor Neurons and Motor Coordination describes the function of the muscle spindle and stretch reflex. These receptors also may be classified as interoceptors because of their location. They include mechanoreceptors that respond to movement or distention of the viscera. These are found in locations such as the mesentery, connective tissue enclosing the organs, and along blood vessels. Nociceptors are found in the viscera, as well. These are capable of responding to noxious mechanical, thermal, and chemical stimuli (Willis & Coggeshall, 1991). Chapter 10 discusses the relationship of these receptors and their afferent fibers to the somatic and autonomic nervous systems. The spinal cord receives impulses from receptors and sends output to effectors via the PNS. Because the PNS transmits this essential information, its components are considered in this section. Thirty-one pairs of spinal nerves exist, and each is formed by the convergence of a dorsal root and a ventral root usually within the intervertebral foramen (IVF). Just distal to this union, each spinal nerve divides into a dorsal ramus (posterior primary division, PPD) and a ventral ramus (anterior primary division, APD) (see Fig. 3-3). The dorsal rami of spinal nerves innervate the zygapophysial joints, skin over the back, and deepest muscles of the neck and back. The ventral rami innervate the extremities and the ventrolateral aspect of the wall of the trunk. Successive thoracic ventral rami retain a clear segmental distribution along the thoracic region. However, the back of the head, the anterior and lateral neck, the shoulders, and upper and lower extremities are innervated by plexuses. Each plexus is formed by a regrouping of adjacent ventral rami. The plexuses are called the cervical, brachial, and lumbosacral plexuses, and each is briefly described here. The cervical plexus is formed by ventral rami of the C1 through C4 cervical nerves. It supplies cutaneous innervation to the dorsolateral part of the head, neck, and shoulder. Motor fibers in this plexus course to the deep cervical muscles, hyoid muscles, diaphragm, and sternocleidomastoid and trapezius muscles (see Chapter 5). The brachial plexus is formed by ventral rami of the C5 through T1 spinal nerves (often with a contribution from C4 and T2). This plexus supplies the upper extremity. (See Chapter 5 for a full description of the brachial plexus and its proximal branches.) Subsequent to the mixing of the ventral rami in the plexus, numerous branches are formed, including five large terminal branches: the axillary, musculocutaneous, radial, ulnar, and median nerves. The axillary nerve (C5 to C6) supplies cutaneous branches to the deltoid region and muscular branches to the deltoid and teres minor muscles. The musculocutaneous nerve (C5 to C7) provides sensory innervation to the anterolateral and posterolateral aspect of the forearm. It supplies motor innervation to the flexors of the arm, which includes the biceps brachii. The radial nerve (C5 to C8, possibly T1) has an extensive area of distribution in both the arm and the forearm. Its cutaneous branches innervate the posterior aspect of the arm and forearm. The superficial radial nerve supplies the skin of the lateral half of the dorsum of the hand and the first three and a half digits, excluding the nails. The radial nerve also innervates the extensor muscles of the upper extremity. The ulnar nerve (C8 and T1, sometimes C7) courses through the arm to supply structures in the forearm and hand. Its motor distribution includes one and a half forearm flexors (ulnar side) and intrinsic hand muscles, including the hypothenar and all interossei muscles and some thenar muscles. Its cutaneous distribution is present only in the hand and encompasses the ulnar half of the hand, including the fifth and medial half of the fourth digits. The median nerve (C6 to C8 and T1, sometimes C5) supplies motor fibers to the forearm flexors (excluding those with ulnar innervation) and some intrinsic hand muscles, including most of the thenar muscles. As with the ulnar nerve, its sensory area of distribution is only in the hand and includes the lateral palmar surface and the first three and a half digits, including the nails (Standring et al., 2008) (Figs. 9-2 and 9-3 and Table 9-2). Table 9-2 Muscles Supplied by Terminal Branches of the Brachial Plexus and the Lumbosacral Plexus ∗This level represents clinically significant contributions in a small portion of the population. FIG. 9-2 Anterior view of the body showing its cutaneous innervation. Left, Dermatomal pattern, which may vary according to different authors. This dermatomal mapping is based on studies by JG Keegan and FV Garrett (1948). Right, Areas of cutaneous peripheral nerve distributions. Note the similarity of cord segment origins between the two sides. FIG. 9-3 Posterior view of the body showing its cutaneous innervation. Left, Dermatomal pattern, which may vary according to different authors. This dermatomal mapping is based on studies by JG Keegan and FV Garrett (1948). Right, Areas of cutaneous peripheral nerve distributions. Note the similarity of cord segment origins between the two sides. The lumbosacral plexus (sometimes described as a lumbar plexus and a sacral plexus) is the third plexus, which is composed of ventral rami L2 through S2 (with contributions from L1 and S3). The major branches of this network are the femoral, obturator, gluteal, sciatic, common fibular (peroneal) (and its branches), and tibial nerves. Cutaneous nerves with large areas of distribution include, but are not limited to, the lateral femoral cutaneous, saphenous, posterior femoral cutaneous, and sural nerves. (Chapters 7 and 8 describe the lumbar plexus and its smaller branches [iliohypogastric and ilioinguinal] and the sacral plexus and its branches.) The common fibular (peroneal) nerve (L4, L5, S1, and S2) courses laterally around the neck of the fibula and divides into two major branches: superficial fibular (peroneal) and deep fibular (peroneal). The superficial fibular (peroneal) nerve supplies muscular branches to the fibularis (peronei) muscles, which are responsible for eversion of the foot, and cutaneous branches to the distal and anterolateral third of the leg and dorsum of the foot (excluding the first digital interspace). The deep fibular (peroneal) nerve sends motor fibers to the anterior leg muscles, which provide dorsiflexion of the foot and extension of the toes. Cutaneous branches supply the skin between the first two toes. The other branch of the sciatic nerve is the tibial nerve (L4, L5, S1, S2, and S3). This nerve provides motor innervation to posterior leg muscles (including the gastrocnemius muscle), which are responsible for plantar flexion of the foot. A branch of the tibial nerve and contributing fibers from the common fibular (peroneal) nerve form the sural nerve. This supplies sensory innervation to the posterior and lateral surfaces of the leg. The tibial nerve divides into medial and lateral plantar nerves at the region of the medial malleolus. These nerves supply motor and sensory innervation to the plantar aspect of the foot (see Figs. 9-2 and 9-3 and Table 9-2). The inferior and superior gluteal nerves innervate muscles moving the hip joint. The inferior gluteal nerve (L5, S1, and S2) is responsible for the motor innervation of the strongest hip extensor, the gluteus maximus. The superior gluteal nerve (L4, L5, and S1) innervates the gluteus medius and minimus muscles and the tensor fascia latae muscle, which are responsible for hip abduction (see Table 9-2). This has been a cursory description of the innervation of major individual muscles and muscle groups and the cutaneous distribution of the major nerves. Because of developmental events, one muscle is innervated by multiple cord segments, and one cord segment may be involved with the innervation of more than one muscle. The intermingling of dorsal root fibers in the plexuses produces a peripheral nerve with an area of distribution that is different from a dermatomal pattern—that is, a pattern indicating the cutaneous area supplied by one dorsal root and its ganglion. However, the origin (cord segments) of a peripheral nerve innervating a particular cutaneous region includes the same cord segments as those supplying the dermatomes of that same area. For example, the lateral femoral cutaneous nerve is formed from cord segments L2 and L3, and its peripheral nerve pattern includes parts of the L2 and L3 dermatomal regions. There is much variation in the dermatome maps that are found in textbooks. Although most maps stem from two major references, Foerster (1933) and/or Keegan and Garrett (1948), other studies have also resulted in mapped dermatomes as well. The variability in dermatomes may be due to two or more dorsal roots innervating an area of the skin. Another possibility may be that the intrathecal intersegmental anastomoses that can occur between dorsal rootlets allow a sensory fiber from one dorsal root ganglion to enter the cord at a different level. A detailed description of these studies as well as a new and unique evidence-based dermatome map has been presented by Lee and colleagues (2008). Knowing a cutaneous nerve’s distribution and its related dermatomal pattern (variable as it may be) as well as both the segmental and the peripheral innervation of major skeletal muscles is very important (see Figs. 9-2 and 9-3). Knowledge of the innervation of muscles and skin at both the PNS and CNS levels is imperative, because a neurologic examination includes the assessment of a patient’s motor functions (reflexes and muscle strength; Table 9-3) and sensory functions. The information gained from this assessment is useful for distinguishing if the lesion is in the CNS or PNS and subsequently for determining the specific location of the lesion along one of these two systems. The multipolar neurons (see Chapter 14, Neural Tissue) that compose the gray matter are subdivided into four groups: motor neurons, the axons of which leave the spinal cord and innervate the effector tissues (skeletal, smooth, and cardiac muscles; glands); tract neurons, the axons of which ascend in the white matter to higher centers; interneurons, which have short processes; and propriospinal neurons, the axons of which provide communication between cord segments. Propriospinal neurons are classified as long, intermediate, or short. Long propriospinal neurons extend the length of the cord in the ventral funiculus and ventral part of the lateral funiculus (see White Matter). Descending propriospinal fibers (axons) course bilaterally and the ascending axons project mostly contralaterally. Short propriospinal neurons extend up to six to eight segments in the ipsilateral lateral funiculus, and intermediate propriospinal neurons course primarily ipsilaterally more than eight segments but less than the cord’s entire length. Most of the propriospinal fibers are located immediately adjacent to the gray matter in the fasciculus proprius, but some travel more laterally in the funiculi. The axons of medial propriospinal neurons are long and include extensive branches. For example, some extend the length of the cord to coordinate the movements of neck and pelvic axial musculature for postural corrections. Laterally placed propriospinal fibers influence neurons innervating more distal muscles, communicate with a smaller number of segments, and branch less extensively. Because the distal musculature is less connected to other muscle groups, these muscles function more independently. This independence permits more diverse somatic motor activities (Ghez & Krakauer, 2000; Standring et al., 2008). Through their communication with neurons in other cord segments, propriospinal neurons are involved not only with the coordination of somatic motor activity, but also with the autonomic innervation of sweat glands, smooth muscle of the vasculature, and viscera such as the bladder and bowel (Standring et al., 2008). In the early 1950s, Rexed (1952) studied feline spinal cords and proposed that the organization of the gray matter formed 10 layers, or laminae. He described lamina I as being located at the tip of the dorsal horn, followed sequentially into the ventral horn by laminae II through IX. Lamina X formed the connecting crossbar of the gray matter, that is, the gray commissure. This organization has been accepted for the human spinal cord as well (Fig. 9-4). Each lamina includes at least one of the four general types of neurons: motor, tract, interneuron, or propriospinal. Each lamina also may be the site of the termination of primary afferents, descending tracts, propriospinal neurons, and interneurons of neighboring laminae. The laminae may vary in size throughout regions of the spinal cord and even may be absent in some regions. Also within each lamina, neurons may be organized into smaller groups, called nuclei or cell columns, based on commonalities such as cell morphology and function. The following is a brief description of each of these laminae. The dorsal horn consists of laminae I through VI. Laminae I through IV, which form the head of the dorsal horn, are the main termination sites of cutaneous afferent fibers and their collateral branches. Lamina V forms the neck, and receives thinly myelinated fibers from the skin, muscle, and viscera. Lamina VI forms the base of the dorsal horn and receives proprioceptive as well as some cutaneous fibers (Standring et al., 2008). Laminae I and II are collectively known as the superficial dorsal horn and are heavily involved with the processing of nociception. The majority of A-delta and C fibers terminate here. Lamina I is also known as the marginal zone of Waldeyer. It is a very thin layer at the dorsolateral tip of the dorsal horn and includes neuron cell bodies that intermingle with thin and thick fibers, a characteristic reflected by its reticular appearance. Most of the primary afferent input into lamina I originates from cutaneous nociceptors and thermoreceptors via A-delta fibers. Additional input is conveyed by C fibers (from nociceptors [peptidergic fibers], thermoreceptors, and histamine-sensitive receptors conveying the sensation of “itch” [Schmelz et al., 1997; Andrew & Craig, 2001; Craig, Zhang, & Blomqvist, 2002]) and by a small group of thinly myelinated muscle, joint, and visceral afferent fibers (Willis & Coggeshall, 1991; Standring et al., 2008). Many neurons within lamina I are considered to be nociceptive specific, whereas others are classified as wide-dynamic-range neurons that respond to both noxious and innocuous stimuli. Thermoreceptive-specific neurons also terminate in lamina I (Han, Zhang, & Craig, 1998) along with numerous interneurons. Tract (projection) neurons originate in lamina I as well, and provide the major output for the superficial dorsal horn. They are the targets of both interneurons and primary afferent neurons. The majority of the axons from these neurons cross the midline although some project bilaterally. Tract neurons are a heterogeneous population and attempts have been made to classify them. In rat studies, data show that 80% of the tract neurons express the main receptor to which substance P binds (neurokinin 1 receptor [NK1R]). Neurons that express NK1R are both large and small, and among those that lack the NK1R is a population that has very large multipolar cell bodies. The dendrites of tract neurons remain in lamina I and based on their morphology these tract neurons may be described as fusiform, pyramidal, or multipolar. Studies using anterograde tracing methods suggest that tract neuron axons terminate in numerous locations within brain stem nuclei (caudal ventrolateral medulla, nucleus of the solitary tract, lateral parabrachial area, and periaqueductal gray) and thalamic nuclei (ventral posterior lateral and posterior). There is widespread collateral branching and some axons may project to at least three locations although evidence suggests that certain neurons preferentially terminate in specific targets. Based on these termination sites, it appears that these tract neurons are involved with numerous functions pertaining to the autonomic nervous system and aspects of cortical nociception processing, including motivation and affect and the sensory-discriminatory component of pain perception (Todd, 2010). Lamina II is known as the substantia gelatinosa of Rolando. The many processes, the presence of small neurons, and the absence of myelinated axons give this layer a gelatinous appearance on close inspection. It is divided into an outer (IIo) and an inner (IIi) layer, the latter showing a lower density of neurons. The primary afferent input into lamina IIo enters by C afferent fibers from thermoreceptors and peptidergic nociceptive C fibers from various tissues and deep regions of the skin. A few A-delta fibers also terminate here. Nonpeptidergic nociceptive C fibers convey input from the epidermis of the skin and terminate in a narrow band within the central part of lamina II. Afferent input from hair follicles (both A-beta and A-delta fibers) and from tactile afferents (A-beta fibers) terminates in the more ventral lamina IIi (Todd, 2010). The neurons of lamina II are almost entirely interneurons (both excitatory and inhibitory), the dendrites of which arborize within the lamina and also project into other laminae. Some interneurons respond to noxious stimuli, whereas others respond to both noxious and innocuous stimuli (Basbaum & Jessell, 2000). Lamina V forms the neck of the dorsal horn. It is a thick layer that is interlaced by bundles of fibers. Primary afferent input comes via A-delta fibers from cutaneous mechanical nociceptors and group III and IV muscle and joint afferents, and nociceptive visceral afferents (Willis & Coggeshall, 1991; Basbaum & Jessell, 2000). Additional input to this lamina (such as input from C fibers) is most likely received via the dendritic projections located within more dorsal laminae. Many neurons within this lamina are wide-dynamic-range tract neurons that are the site of viscerosomatic convergence for visceral referred pain (Benarroch et al., 1999), whereas others are interneurons and propriospinal neurons. Cortical and subcortical fibers terminate in lamina V as well, indicating its involvement in motor function. In summary, the first six laminae that comprise the dorsal horn are the major receiving areas for sensory information. Pain and temperature input appears to terminate primarily in superficial layers; mechanical types of stimuli terminate in the middle region; and proprioceptive input ends in the base of the dorsal horn near the motor regions. Each primary afferent fiber has many collateral branches that synapse in the circuitry of more than one lamina and feed into more than one ascending pathway. For example, one Ia afferent fiber from a neuromuscular spindle may have 500 or more branches terminating within the gray matter of the cord (Nolte, 2002). Many of the laminae communicate with each other via profuse dendritic branching and the axonal projections of their interneurons. This provides a mechanism by which incoming sensory signals may be processed and modified (modulated) before ascending to higher centers. This modulation occurs at the level of the synapse between presynaptic and postsynaptic neurons and involves not only the release of neurotransmitters and neuromodulators but also the presence of the different receptors with which these chemicals bind. This is exemplified by studies of the nociceptive interneurons in lamina II that show that numerous chemicals can modulate synaptic transmission. Dorsal horn interneurons: Interneurons in the dorsal horn play a key role in modifying sensory information and ongoing movements, as well as controlling all reflexive movements. They form the neuronal circuitry that links sensory fibers with the tract neurons that provide the major output of the dorsal horn. Numerous studies have described the interneurons located in the superficial dorsal horn (Todd, 2010). There are two classes of interneurons: excitatory, which utilize the neurotransmitter glutamine; and inhibitory, which use gamma-aminobutyric acid (GABA) and/or glycine as their neurotransmitter. Since most primary afferent terminals exhibit axoaxonic synapses, it has been suggested that one function of GABAergic interneurons is to provide presynaptic inhibitory input to afferent fibers. Interneurons in lamina II have been classified as either islet, central, vertical, or radial on the basis of their dendritic morphology. Studies using immunocytochemistry methods indicate that the islet cells are GABAergic, radial cells are glutaminergic, vertical cells are mostly GABAergic but can be glutaminergic and central cells which can be either type. An additional 30% have yet to be classified. Lamina I interneurons have been described as pyramidal, fusiform, or multipolar. However, projection neurons are present in this lamina and may have been inadvertently classified as part of the population because they have not been distinguished from the interneurons. The extensive branching of the afferent inputs to the spinal cord results in simultaneous activation of many types of interneurons. The activity of these interneurons and their subsequent synapses on cells in the ventral horn (motor systems), ascending tracts (sensory perception of stimuli), descending tracts (sensitivity of motor and sensory pools), and other interneurons in the spinal cord controls the overall sensitivity and general responsiveness of all neurons in the spinal cord. The balance between the firing of excitatory and inhibitory interneurons is critical in the maintenance of normal sensory function. Disruptions in the transmission from interneuronal circuits can result in allodynia (see Appendix II) or the development and maintenance of inflammation and neuropathic pain (Todd, 2010). In addition to the input from afferent fibers, the spinal interneurons receive input from other sources including descending fibers from higher centers. The superficial dorsal horn receives input from serotonergic fibers, noradrenergic fibers, and GABAergic fibers, all of which originate in different brain stem nuclei (Todd, 2010). Additional sources that influence spinal interneurons include ventral horn alpha motor neurons, local (at the level) interneurons, short propriospinal neurons (from one or two levels above or below), and long propriospinal neurons (ascending and descending between all levels of the cord). This vast array of interconnections among and between the various interneurons also contributes to the general responsiveness of the spinal cord to both afferent (sensory or reflexive) and efferent (motor or voluntary) stimuli. The interconnections between the interneurons are mutually inhibitory, meaning that only one set of pathways can be active at any time. When one particular response pathway and its corresponding interneurons are activated, all other interneuronal pathways typically are inhibited. This allows the spinal cord to respond selectively to any input, either afferent or efferent, without inappropriately activating other antagonistic pathways that might hinder the appropriate response. In addition, interneurons can modulate or alter the normal output of higher-order neurons. One example of this is related to the gate control theory of pain (see Fig. 11-6), in which nonnociceptive afferents reduce the output of second-order pain fibers via inhibitory interneurons in the dorsal horn. Therefore interneurons are the key building blocks of all spinal reflexes, and can control or modulate most afferent and efferent information at the level of the spinal cord (see the Spinal Motor Neurons and Motor Coordination section later in this chapter). Relationship between the dorsal horn and trigeminal nerve: The dorsal horn of the upper three or four cervical cord segments has an interesting relationship with the trigeminal nerve. The trigeminal nerve (cranial nerve V) provides sensory innervation to the skin of the face and other structures, including the paranasal sinuses, cornea, temporomandibular joint, and oral and nasal mucosae. The trigeminal nerve also supplies motor fibers to the muscles of mastication and several other small muscles of the head. The afferent fibers of the trigeminal nerve enter the brain stem at the level of the pons and synapse in a nuclear column that extends from the midbrain through the pons and medulla and into the upper cervical cord segments. The portion of the nuclear column located in the medulla and upper cervical cord segments is known as the spinal trigeminal nucleus (Fig. 9-5). Afferent fibers conveying pain and temperature (and some touch) that enter the brain stem within the trigeminal nerve (and also in the facial and glossopharyngeal nerves) descend as the spinal trigeminal tract and synapse in this nucleus. The most inferior portion of the spinal trigeminal nucleus is the nucleus caudalis. This nuclear region continues from the caudal medulla into the upper three or four cervical cord segments and blends with laminae of the dorsal horn of those segments (Carpenter, 1991; Standring et al., 2008). This area of gray matter that is the site of convergence of trigeminal and cervical afferents is also called the trigeminocervical (Bogduk, 1992; Biondi, 2001) or trigeminospinal nucleus. The descending spinal trigeminal tract fibers also continue into the dorsolateral tract of Lissauer in the upper cervical segments. Afferent fibers conveying similar information and traveling in dorsal roots of the upper three or four cervical nerves also synapse in these same laminae. In fact, some of these cervical dorsal root afferents may ascend into the rostral medulla and synapse in the spinal trigeminal nucleus (Abrahams, 1989). The relationship between the dorsal horn and trigeminal system is clinically significant (Pollman, Keidel, & Pfaffenrath, 1997; Biondi, 2000, 2001; Bogduk, 2001; Bogduk & Govind, 2009). The upper three cervical spinal nerves innervate the muscles (including the trapezius and sternocleidomastoid muscles), ligaments, and joints such as the zygapophysial joints in the region of the upper three cervical vertebral segments, C2-3 intervertebral discs, vertebral and internal carotid arteries, and dura mater of the upper spinal cord and posterior cranial fossa of the skull. Second-order neurons in the dorsal horn, which project to the brain, receive nociceptive afferents traveling in the C1 to C3 spinal nerves from these structures and from afferents coursing in adjacent cervical spinal nerves. Afferents from lower cervical spinal nerves do not appear to have a direct anatomic relationship with these second-order neurons (Bogduk & Govind, 2009). Because of the convergence of cervical afferents from the occiput with cervical spinal afferents in the dorsal horn, a cervical-cervical pain referral pattern may occur. In this case nociceptive input from upper cervical pain generators is perceived in the region of the head that is innervated by cervical nerves (e.g., external occiput or the posterior cranial fossa [Bogduk, 2004
Neuroanatomy of the Spinal Cord
Peripheral Nervous System
Cutaneous Receptors
Muscle, Tendon, and Joint Receptors
Visceral Receptors
Peripheral Nerves
Internal Organization of the Spinal Cord
Laminae I through VI (Dorsal Horn)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree