CHAPTER 3 Nervous system
The nervous system has two major divisions, the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord and contains the majority of neuronal cell bodies. The PNS includes all nervous tissue outside the central nervous system and is subdivided into the cranial and spinal nerves, peripheral autonomic nervous system (ANS) (including the enteric nervous system of the gut wall, ENS) and special senses (taste, olfaction, vision, hearing and balance). It is composed mainly of the axons of sensory and motor neurones that pass between the CNS and the body. However, the ENS contains as many intrinsic neurones in its ganglia as the entire spinal cord, is not connected directly to the CNS, and may be considered separately as a third division of the nervous system (Gershon 1998).
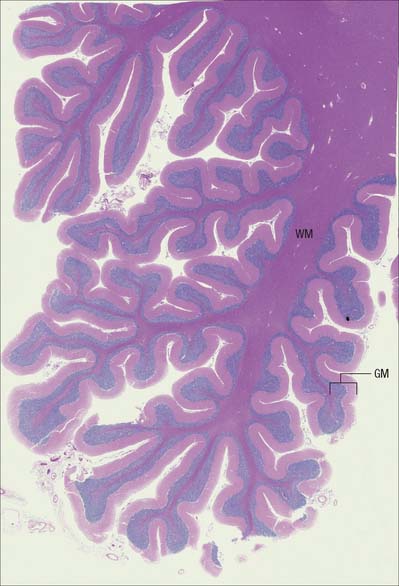
The CNS is derived from the neural tube (Ch. 24). The cell bodies of neurones are often grouped together in discrete areas termed nuclei, or they may form more extensive layers or masses of cells; collectively they constitute grey matter. Neuronal dendrites and synaptic contacts are mostly confined to areas of grey matter, and they form part of its meshwork of neuronal and glial processes termed the neuropil. Their axons join bundles of nerve fibres that tend to be grouped separately to form tracts. In the spinal cord, cerebellum, cerebral cortices (Chs 20, 22) and some other areas, concentrations of tracts constitute the white matter, so called because the axons are often ensheathed in lipid-rich sheaths of myelin which is white when fresh (Fig. 3.1).
The PNS is composed of the efferent axons (fibres) of motor neurones situated inside the CNS, and the cell bodies of sensory neurones (grouped together as ganglia) and their afferent processes. Sensory cells in dorsal root ganglia give off both centrally and peripherally directed processes: there are no synapses on their cell bodies. Also included are ganglionic neurones of the ANS, which receive synaptic contacts from the peripheral fibres of preganglionic autonomic neurones whose cell bodies lie within the CNS. Neuronal cell bodies in peripheral ganglia are all derived embryologically from cells which migrate from the neural crest (p. 201).
The nervous system contains large populations of non-neuronal cells, neuroglia or glia, which, whilst not electrically active in the same way, are responsible for creating and maintaining an appropriate environment in which neurones can operate efficiently. Indeed, it is now known that two-way communication between neurones and glial cells is essential for normal neural activity (reviewed in Fields & Stevens-Graham 2002). In the CNS, glia outnumber neurones by 10–50 times and consist of microglia and macroglia. Macroglia are further subdivided into three main types, oligodendrocytes, astrocytes and ependymal cells. The principal glial cell of the PNS is the Schwann cell. Satellite cells surround each neuronal soma in ganglia.
NEURONES
Most of the neurones in the CNS are either clustered into nuclei, columns or layers, or dispersed within grey matter. Neurones of the PNS are confined to ganglia. Irrespective of location, neurones share many general features, which are discussed here in the context of central neurones. Special characteristics of ganglionic neurones and their adjacent tissues are discussed on page 55–6.
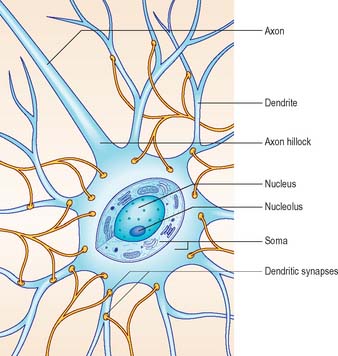
Neurones exhibit great variability in their size (cell bodies range from 5 to 100 μm diameter) and shapes. Their surface areas are extensive because most neurones display numerous narrow branched cell processes. They usually have a rounded or polygonal cell body (perikaryon or soma). This is a central mass of cytoplasm which encloses a nucleus and gives off long, branched extensions, with which most intercellular contacts are made. Typically, one of these processes, the axon, is much longer than the others, the dendrites (Fig. 3.2). Dendrites conduct electrical signals towards a soma whereas axons conduct impulses away from it.
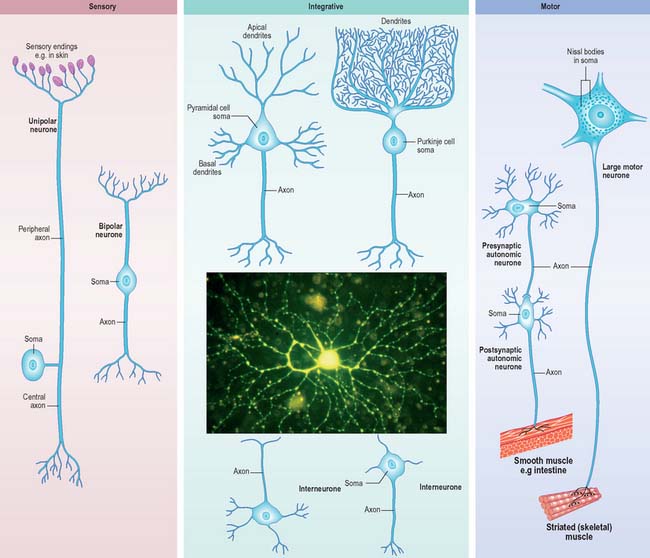
Neurones can be classified according to the number and arrangement of their processes. Multipolar neurones (Fig. 3.3) are common: they have an extensive dendritic tree which arises from either a single primary dendrite or directly from the soma, and a single axon. Bipolar neurones, which typify neurones of the special sensory systems, e.g. the retina (p. 697), have only a single dendrite which emerges from the soma opposite the axonal pole. Unipolar neurones which transmit general sensation, e.g. dorsal root ganglion neurones, have a single short process which bifurcates into peripheral and central processes (p. 55). This arrangement arises by the fusion of the proximal axonal and dendritic processes of a bipolar neurone during development: they may also, therefore, be termed pseudounipolar. Neurones are also classified according to whether their axons terminate locally on other neurones (interneurones), or transmit impulses over long distances, often to distinct territories via defined tracts (projection neurones).
Neurones are postmitotic cells and, with few exceptions, they are not replaced when lost.
SOMA
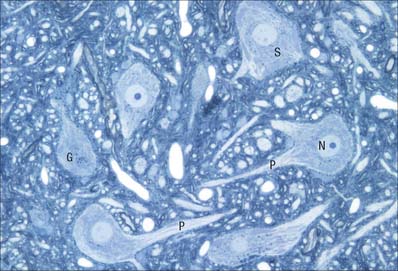
The cytoplasm of a typical soma (Fig. 3.2) is rich in rough and smooth endoplasmic reticulum and free polyribosomes, indicating a high level of protein synthetic activity. Free polyribosomes often congregate in large groups associated with the rough endoplasmic reticulum. These aggregates of RNA-rich structures are visible by light microscopy as basophilic Nissl bodies or granules. They are more obvious in large, highly active cells, such as spinal motor neurones (Fig. 3.4), which contain large stacks of rough endoplasmic reticulum and polyribosome aggregates. Maintenance and turnover of cytoplasmic and membranous components are necessary in all cells: the huge total volume of cytoplasm within the soma and processes of many neurones requires a considerable commitment of protein synthetic machinery. Neurones also synthesize other proteins (enzyme systems, etc.) involved in the production of neurotransmitters and in the reception and transduction of incoming stimuli. Various transmembrane channel proteins and enzymes are located at the surfaces of neurones where they are associated with movements of ions. The apparatus for protein synthesis (including RNA and ribosomes) occupies the soma and dendrites, but is usually absent from axons.
The neuronal cytoskeleton is a prominent feature of its cytoplasm and gives shape, strength and support to the dendrites and axons. A number of neurodegenerative diseases are characterized by abnormal aggregates of cytoskeletal proteins (reviewed in Cairns et al 2004). Neurofilaments (the intermediate filaments of neurones) and microtubules are abundant in the soma and along dendrites and axons: the proportions vary with the type of neurone and cell process. Bundles of neurofilaments constitute neurofibrils which can be seen by light microscopy in silver stained or immunolabelled sections. Neurofilaments are heteropolymers of proteins assembled from three polypeptide subunits, NF-L (68 kilodaltons [kDa]), NF-M (160 kDa) and NF-H (200 kDa). NF-M and NF-H have long C-terminal domains which project as side arms from the assembled neurofilament and bind to neighbouring filaments. They can be heavily phosphorylated, particularly in the highly stable neurofilaments of mature axons, and are thought to give axons their tensile strength. Some axons are almost filled by neurofilaments.
Microtubules are important in axonal transport, although dendrites usually have more microtubules than axons. Centrioles persist in mature postmitotic neurones, where they are concerned with the generation of microtubules rather than cell division. Centrioles are associated with cilia on the surfaces of developing neuroblasts. Their significance, other than at some sensory endings (e.g. the olfactory mucosa, p. 553), is not known.
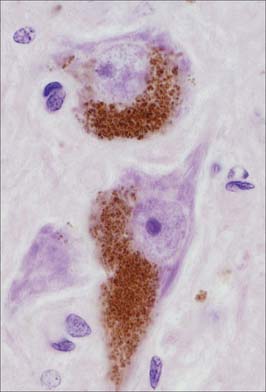
Pigment granules (Fig. 3.5) appear in certain regions, e.g. neurones of the substantia nigra contain neuromelanin, probably a waste product of catecholamine synthesis. In the locus coeruleus a similar pigment, rich in copper, gives a bluish colour to the neurones. Some neurones are unusually rich in certain metals which may form a component of enzyme systems, e.g. zinc in the hippocampus and iron in the oculomotor nucleus. Ageing neurones, especially in spinal ganglia, accumulate granules of lipofuscin (senility pigment) in residual bodies, which are lysosomes packed with partially degraded lipoprotein material (corpora amylaceae).
DENDRITES
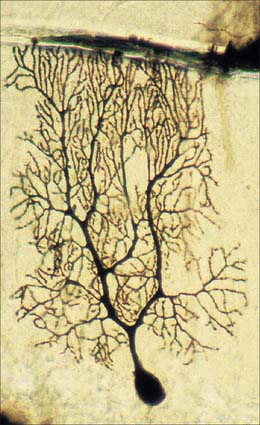
Dendrites are highly branched, usually short processes which project from the soma (Fig. 3.2). The branching patterns of many dendritic arrays are probably established by random adhesive interactions between dendritic growth cones and afferent axons that occur during development. There is an overproduction of dendrites in early development, and this is pruned in response to functional demand as the nervous system matures and information is processed through the dendritic tree. There is evidence that dendritic trees may be plastic structures throughout adult life, expanding and contracting as the traffic of synaptic activity varies through afferent axodendritic contacts (for review see Wong & Ghosh 2002). Groups of neurones with similar functions have a similar stereotypic tree structure (Fig. 3.6), suggesting that the branching patterns of dendrites are important determinants of the integration of the afferent inputs that converge on the tree.
Dendrites differ from axons in many respects. They represent the afferent rather than the efferent system of the neurone, and receive both excitatory and inhibitory axodendritic contacts. They may also make dendrodendritic and dendrosomatic connections (see Fig. 3.9), some of which are reciprocal. Synapses occur either on small projections called dendritic spines or on the smooth dendritic surface. Dendrites contain ribosomes, smooth endoplasmic reticulum, microtubules, neurofilaments, actin filaments and Golgi complexes. Their neurofilament proteins are poorly phosphorylated and their microtubules express the microtubule-associated protein (MAP)-2 almost exclusively in comparison with axons.
The shapes of dendritic spines range from simple protrusions to structures with a slender stalk and expanded distal end. Most spines are not more than 2 μm long, and have one or more terminal expansions; they can also be short and stubby, branched or bulbous. Free ribosomes and polyribosomes are concentrated at the base of the spine. Ribosomal accumulations near synaptic sites provide a mechanism for activity- dependent synaptic plasticity through the local regulation of protein synthesis.
AXONS
The axon originates either from the soma or from the proximal segment of a dendrite, at a specialized region free of Nissl granules, the axon hillock (Fig. 3.2). Action potentials are initiated here. The axonal plasma membrane (axolemma) is undercoated at the hillock by a concentration of cytoskeletal molecules, including spectrin and actin fibrils, which are important in anchoring numerous voltage-sensitive channels to the membrane. The axon hillock is unmyelinated and often participates in inhibitory axo-axonal synapses. It is unique because it contains ribosomal aggregates immediately below the postsynaptic membrane.
In the CNS, small, unmyelinated axons lie free in the neuropil, whereas in the PNS they are embedded in Schwann cell cytoplasm. Myelin, which is formed around almost all axons >2 μm diameter by oligodendrocytes in the CNS and Schwann cells in the PNS, begins at the distal end of the axon hillock. Nodes of Ranvier are specialized constricted regions of myelin-free axolemma where action potentials are generated and where an axon may branch. In both CNS and PNS, the territory of a myelinated axon between adjacent nodes is called an internode: the region close to a node, where the myelin sheath terminates, is called the paranode. Myelin thickness and internodal lengths are, in general, positively correlated with axon diameter. The density of sodium channels in the axolemma is highest at nodes of Ranvier, and very low along internodal membranes: sodium channels are spread more evenly within the axolemma of unmyelinated axons. Fast potassium channels are present in the paranodal regions of myelinated axons. Fine processes of glial cytoplasm (astrocytic in the CNS, Schwann cell in the PNS) surround the nodal axolemma.
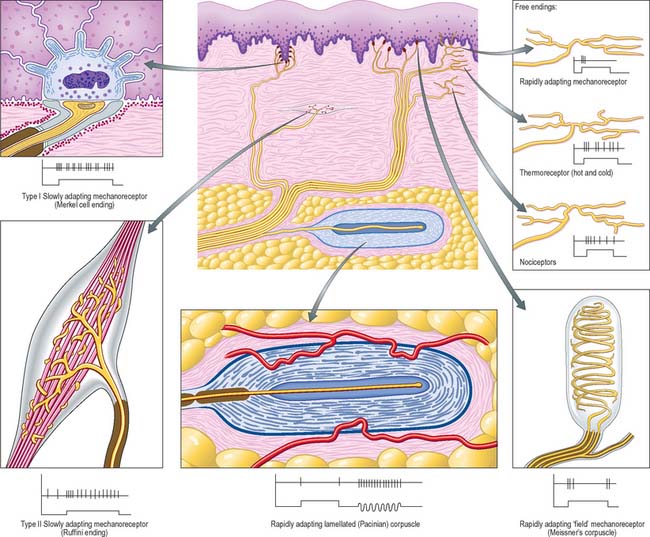
The terminals of an axon are unmyelinated and most expand into presynaptic boutons which may form connections with axons, dendrites, neuronal somata or, in the periphery, muscle fibres, glands and lymphoid tissue. Exceptions include the free afferent sensory endings in e.g. the epidermis, which are unspecialized structurally, and the peripheral terminals of afferent sensory fibres with encapsulated endings (see Fig. 3.30). Axon terminals may themselves be contacted by other axons, forming axoaxonal presynaptic inhibitory circuits. Further details of neuronal microcircuitry are given in Kandel et al (2000).
Axoplasmic flow
Rapid flow depends on microtubules. Vesicles with side projections line up along microtubules and are transported along them by their side-arms. Two microtubule-based motor proteins with ATPase activity are involved in fast transport: kinesin family proteins are responsible for the fast component of anterograde transport, and cytoplasmic dynein is responsible for retrograde transport. Microtubule-dependent axonal transport is reviewed in Guzik and Goldstein (2004). Retrograde transport mediates the movement of neurotrophic viruses, e.g. herpes zoster, rabies and polio, from peripheral terminals, and their subsequent concentration in the neuronal soma.
SYNAPSES
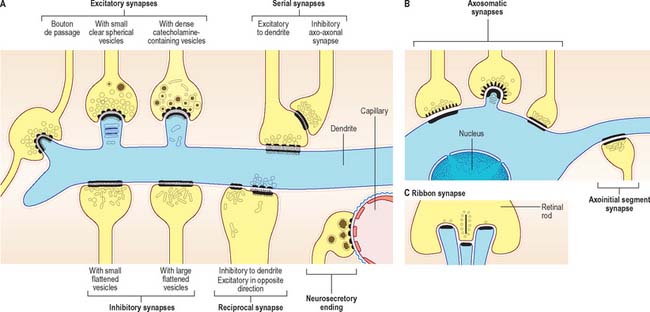
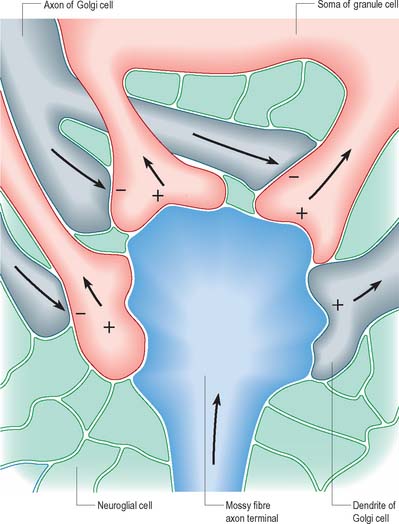
The patterns of axonal termination vary considerably. A single axon may synapse with one neurone (e.g. climbing fibres ending on cerebellar Purkinje neurones), or more often with many neurones (e.g. cerebellar parallel fibres, which provide an extreme example of this phenomenon (p. 302). In synaptic glomeruli, e.g. in the olfactory bulb and cerebellum, and synaptic cartridges, groups of synapses between two or many neurones form interactive units encapsulated by neuroglia (Fig. 3.7).
Classification of chemical synapses
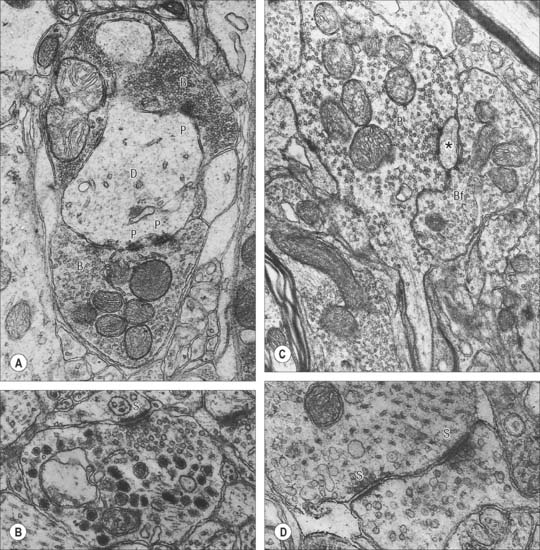
Chemical synapses have an asymmetric structural organization (Fig. 3.8 and Fig. 3.9) in keeping with the unidirectional nature of their transmission. Typical chemical synapses share a number of important features. They all display an area of presynaptic membrane apposed to a corresponding postsynaptic membrane: the two are separated by a narrow (20–30 nm) gap, the synaptic cleft. Synaptic vesicles containing neurotransmitter lie on the presynaptic side, clustered near a dense plaque on the cytoplasmic aspect of the presynaptic membrane. A corresponding region of submembrane density is present on the postsynaptic side. Together these define the active zone, the area of the synapse where neurotransmission takes place.
Chemical synapses can be classified according to a number of different parameters including the neuronal regions forming the synapse, their ultrastructural characteristics, the chemical nature of their neurotransmitter(s) and their effects on the electrical state of the postsynaptic neurone. The following classification is limited to associations between neurones. Neuromuscular junctions share many (though not all) of these parameters, and are often referred to as peripheral synapses. They are described separately on page 63.
Synapses can occur between almost any surface regions of the participating neurones. The most common type occurs between an axon and either a dendrite or a soma, when the axon is expanded as a small bulb or bouton (Fig. 3.8, Fig. 3.9). This may be a terminal of an axonal branch (terminal bouton) or one of a row of bead-like endings, when the axon makes contact at several points, often with more than one neurone (bouton de passage). Boutons may synapse with dendrites, including dendritic spines or the flat surface of a dendritic shaft, a soma (usually on its flat surface, but occasionally on spines), the axon hillock and the terminal boutons of other axons.
Ultrastructurally, synaptic vesicles may be internally clear or dense, and of different size (loosely categorized as small or large) and shape (round, flat or pleomorphic, i.e. irregularly shaped). The submembranous densities may be thicker on the postsynaptic than on the presynaptic side (asymmetric synapses), or equivalent in thickness (symmetrical synapses). Synaptic ribbons are found at sites of neurotransmission in the retina and inner ear. They have a distinctive morphology, in that the synaptic vesicles are grouped around a ribbon- or rod-like density orientated perpendicular to the cell membrane (Fig. 3.9).
Synaptic boutons make obvious close contacts with postsynaptic structures, but many other terminals lack specialized contact zones. Areas of transmitter release occur in the varicosities of unmyelinated axons, where effects are sometimes diffuse, e.g. the aminergic pathways of the basal ganglia and in autonomic fibres in the periphery. In some instances, such axons may ramify widely throughout extensive areas of the brain and affect the behaviour of very large populations of neurones, e.g. the diffuse cholinergic innervation of the cerebral cortices. Pathological degeneration of these pathways can therefore cause widespread disturbances in neural function.
Neurosecretory endings found in various parts of the brain and in neuroendocrine glands and cells of the dispersed neuroendocrine system (see below; p. 31) share many features with presynaptic boutons. They all contain peptides or glycoproteins within dense-core vesicles. The latter are of characteristic size and appearance: they are often ellipsoidal or irregular in shape, and relatively large, e.g. oxytocin and vasopressin vesicles in the neurohypophysis may be up to 200 nm across.
Mechanisms of synaptic activity
Neurohormones
Neurohormones are included in the class of molecules with neurotransmitter-like activity. They are synthesized in neurones and released into the blood circulation by exocytosis at synaptic terminal-like structures. As with classic endocrine gland hormones, they may act at great distances from their site of secretion. Neurones secrete into the CSF or local interstitial fluid to affect other cells, either diffusely or at a distance. To encompass this wide range of phenomena the general term neuromediation has been used, and the chemicals involved are called neuromediators.
Neurotransmitter molecules
Monoamines
Dopamine is a neuromediator of considerable clinical importance, found mainly in neurones with cell bodies in the telencephalon, diencephalon and mesencephalon. A major dopaminergic neuronal population in the midbrain constitutes the substantia nigra, so called because its cells contain neuromelanin, a black granular byproduct of dopamine synthesis. Dopaminergic endings are particularly numerous in the corpus striatum, limbic system and cerebral cortex. Structurally, dopaminergic synapses contain numerous dense-cored vesicles that resemble those containing noradrenaline. Pathological reduction in dopaminergic activity has widespread effects on motor control, affective behaviour and other neural activities, as seen in Parkinson’s syndrome (Ch. 22).
Neuropeptides
Substance P (SP) was the first of the peptides to be characterized as a gastrointestinal neuromediator and is considered to be the prototypic neuropeptide. It is an 11-amino acid polypeptide that belongs to the tachykinin neuropeptide family, and is a major neuromediator in the brain and spinal cord. Contained within large granular synaptic vesicles, SP is found in approximately 20% of dorsal root and trigeminal ganglion cells, in particular in small nociceptive neurones, and in some fibres of the facial, glossopharyngeal and vagal nerves. Within the CNS, SP is present in several apparently unrelated major pathways, and has been described in the limbic system, basal ganglia, amygdala and hypothalamus. Its known action is prolonged postsynaptic excitation, particularly from nociceptive afferent terminals, which sustains the effects of noxious stimuli. SP is one of the main neuropeptides that trigger an inflammatory response in the skin and has also been implicated in the vomiting reflex, changes in cardiovascular tone, stimulation of salivary secretion, smooth muscle contraction, and vasodilation.
CENTRAL GLIA
Glial (neuroglial) cells vary considerably in type and number in different regions of the CNS. There are two major groups, macroglia (astrocytes and oligodendrocytes) and microglia, classified according to origin. Macroglia arise within the neural plate, in parallel with neurones, and constitute the great majority of glial cells. Their functions are diverse and are now known to extend beyond a passive supporting role (reviewed in He & Sun, 2007). Microglia are smaller cells, generally considered to be monocytic in origin, and are derived from haemopoietic tissue (see Fig. 3.21).
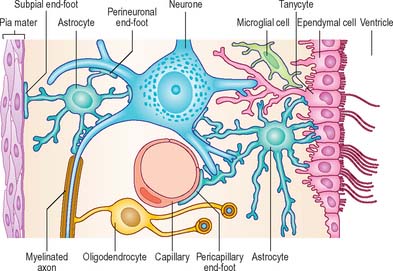
ASTROCYTES
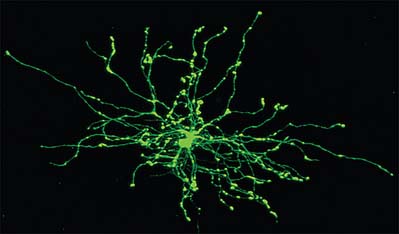
Astrocytes are star-shaped glia (Fig. 3.10). Their processes, which ramify through the entire central neuropil (Fig. 3.11), are coupled functionally at gap junctions, forming an interconnected network which ensheathes all neurones, other than at synapses and along the myelinated segments of axons. Some astrocyte processes terminate as end-feet on the basal lamina of blood vessels, and at the pial surface, where they form the glia limitans (glial limiting membrane).
Astrocytes are now thought to provide a network of communication, providing integration and regulation of function in the brain (reviewed in Fields 2004, Volterra & Meldolesi 2005). One form of communication, with other astrocytes, is via interconnecting low resistance gap junctional complexes. There is recent evidence that astrocytic (and other neural) gap junctions may be formed by a novel family of proteins, the pannexins (see p. 7). They signal to each other using intracellular calcium wave propagation, triggered by synaptically-released glutamate. Signalling presumably co-ordinates astrocyte functions that are essential for efficient neuronal activity, such as ion (particularly potassium) buffering; neurotransmitter uptake and metabolism (e.g. of excess glutamate, which is excitotoxic); membrane transport; the secretion of peptides, amino acids, trophic factors etc. The complexity of astrocytic function and dysfunction in neurological disorders is reviewed in Seifert et al (2006).