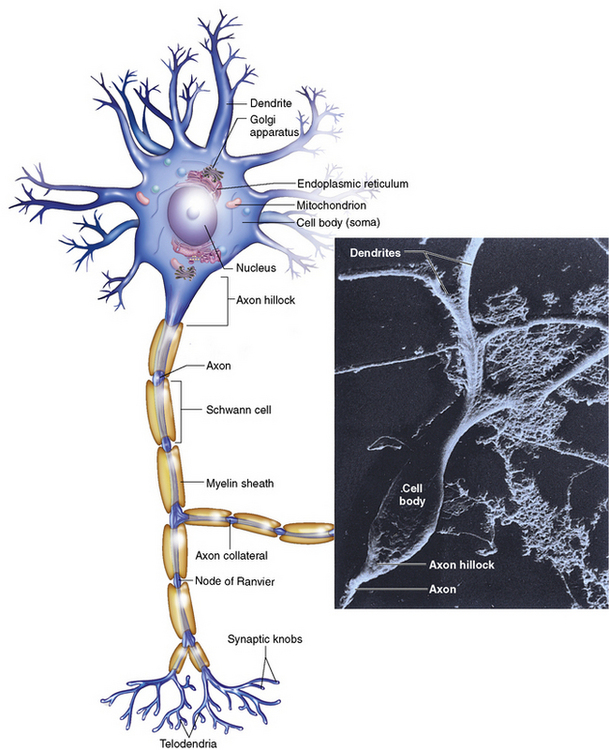
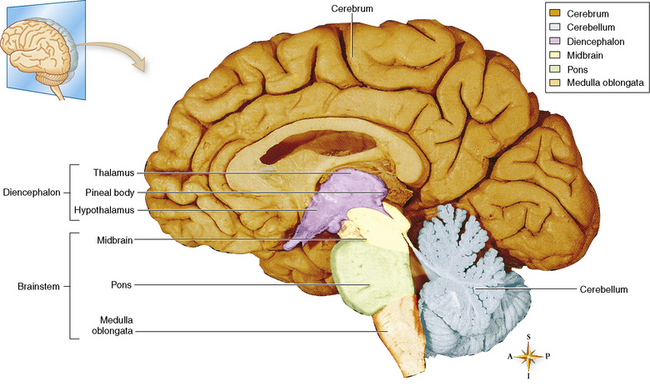
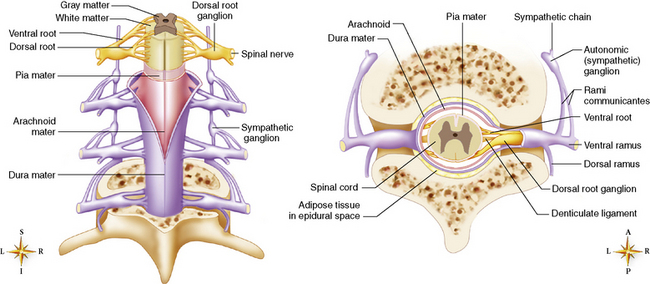
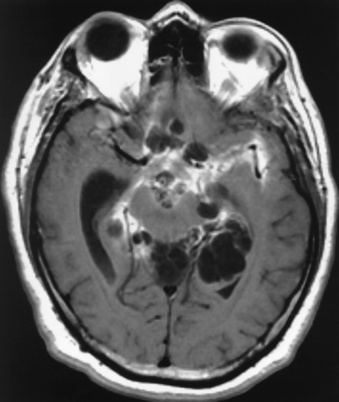
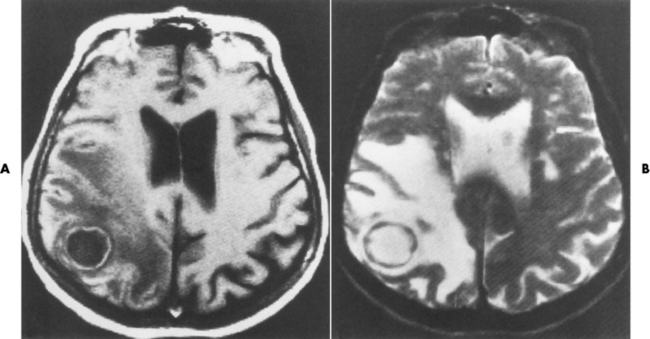
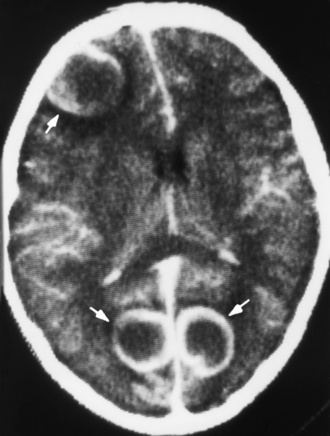
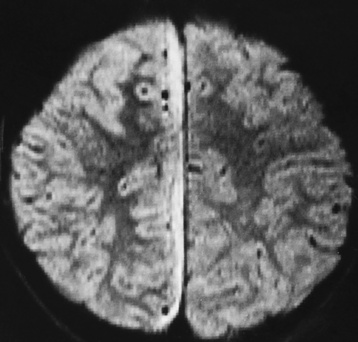
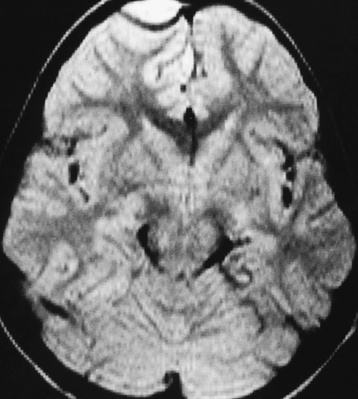
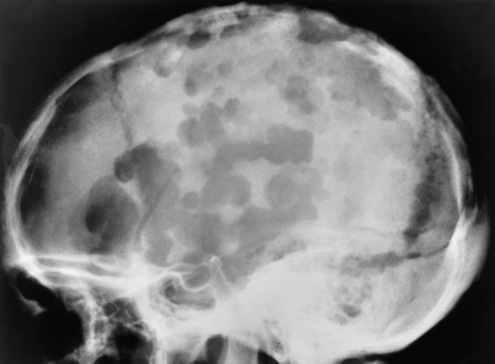
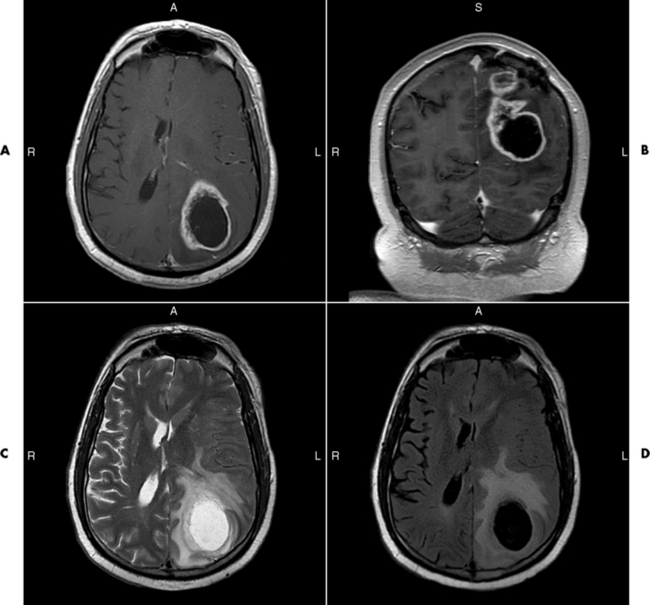
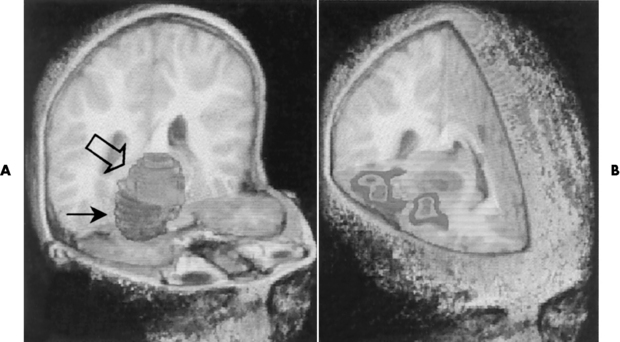
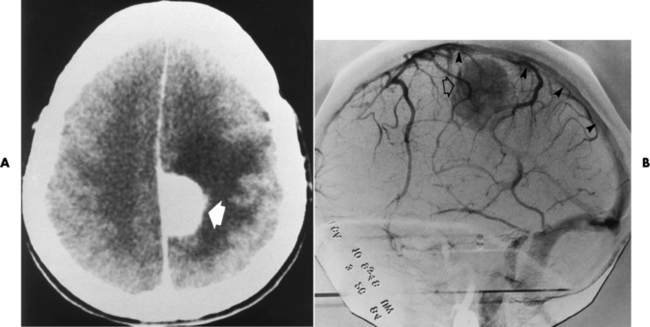
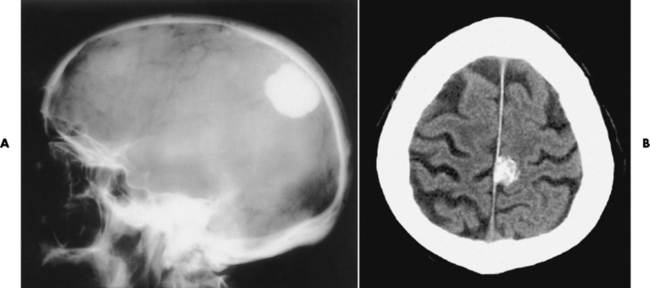
Chapter 8 After reading this chapter, the reader will be able to: 1 Define and describe all bold-faced terms in this chapter 2 Describe the physiology of the nervous system 3 Identify anatomic structures on both diagrams and radiographs of the skull and nervous system 4 Differentiate the various pathologic conditions affecting the skull and nervous system, and their radiographic manifestations The divisions of the nervous system can be classified by location or by the type of tissue supplied by the nerve cells in the division. The central nervous system (CNS) consists of the brain and spinal cord. The remaining neural structures, including 12 pairs of cranial nerves, 31 pairs of spinal nerves, autonomic nerves, and ganglia, make up the peripheral nervous system (PNS). The PNS consists of afferent and efferent neurons. Afferent (sensory) neurons conduct impulses from peripheral receptors to the CNS. Efferent (motor) neurons conduct impulses away from the CNS to the peripheral effectors. The somatic nervous system supplies the striated skeletal muscles, whereas the autonomic nervous system supplies smooth muscle, cardiac muscle, and glandular epithelial tissue. The basic unit of the nervous system is the neuron, or nerve cell (Figure 8-1). A neuron consists of a cell body and two types of long, threadlike extensions. A single axon leads from the nerve cell body, and one or more dendrites lead toward it. Axons are insulated by a fatty covering called the myelin sheath, which increases the rate of transmission of nervous impulses. Deterioration of this fatty myelin sheath (demyelination) is a characteristic abnormality in multiple sclerosis. In involuntary reactions the impulse conduction route to and from the CNS is termed a reflex arc. Voluntary actions are commonly a reaction due to stimulation of a combination of sensors. The basic reflex arc consists of an afferent, or sensory, neuron, which conducts impulses to the CNS from the periphery; and an efferent, or motor, neuron, which conducts impulses from the CNS to peripheral effectors (muscles or glandular tissue). Impulses pass from one neuron to another at a junction called the synapse. Transmission at the synapse is a chemical reaction in which the termini of the axon release a neurotransmitter substance that produces an electrical impulse in the dendrites of the next axon. Once the neurotransmitter has accomplished its task, its activity rapidly terminates so that subsequent impulses pass along this same route. The largest part of the brain is the cerebrum, which consists of two cerebral hemispheres (Figure 8-2). The surface of the cerebrum is highly convoluted with elevations called gyri and shallow grooves called sulci. Deeper grooves called fissures divide each cerebral hemisphere into lobes. The outer portion of the cerebrum, termed the cortex, consists of a thin layer of gray matter where the nerve cell bodies are concentrated. The inner area consists of white matter, which is composed of the nerve fiber tracts. The two cerebral hemispheres are connected by a mass of white matter called the corpus callosum. These extensive bundles of nerve fibers lie in the midline just above the roofs of the lateral ventricles. Deep within the white matter are a few islands of gray matter that are collectively called the basal ganglia. These structures help control position and automatic movements and consist of the caudate nuclei, the globus pallidus, and the putamen. Between the cerebrum and spinal cord lies the brainstem, which is composed of (from top down) the midbrain (mesencephalon), the pons, and the medulla (see Figure 8-2). In addition to performing sensory, motor, and reflex functions, the brainstem contains the nuclei of the 12 cranial nerves and the vital centers controlling cardiac, vasomotor, and respiratory function. Centers in the medulla are responsible for such nonvital reflexes as vomiting, coughing, sneezing, hiccupping, and swallowing. The cerebellum, the second largest part of the brain, is located just below the posterior portion of the cerebrum (see Figure 8-2). It is composed of two large lateral masses: the cerebellar hemispheres and a central section (vermis) that resembles a worm coiled on itself. The cerebellum acts with the cerebral cortex to produce skilled movements by coordinating the activities of groups of muscles. It coordinates skeletal muscles used in maintaining equilibrium and posture by functioning below the level of consciousness to make movements smooth rather than jerky, steady rather than trembling, and efficient and coordinated rather than ineffective and awkward. Therefore, cerebellar disease produces such characteristic symptoms as ataxia (muscle incoordination), tremors, and disturbances of gait and equilibrium. The diencephalon lies between the cerebrum and the midbrain (see Figure 8-2). It consists of several structures located around the third ventricle, primarily the thalamus and hypothalamus. The thalamus primarily functions as a relay station that receives and processes sensory information of almost all kinds of sensory impulses before sending this information on to the cerebral cortex. The tiny hypothalamus is an extremely complex structure that functions as a link between the mind and body and is the site of “pleasure” or “reward” centers for such primary drives as eating, drinking, and mating. It plays a major role in regulating the body’s internal environment by coordinating the activities of the autonomic nervous system and secreting the releasing hormones that control the secretion of hormones by the anterior and posterior portions of the pituitary gland. The hypothalamus is also important in helping to maintain a normal body temperature and in keeping the individual in a waking state. The delicate, yet vital, brain and spinal cord are protected by two layers of coverings. The outer bony coverings are the cranial bones of the skull encasing the brain and the vertebrae surrounding the spinal cord. The inner coverings consist of three distinct layers of meninges (Figure 8-3). The innermost layer adhering to the outer surface of the brain and spinal cord is the transparent pia mater, and the tough outermost covering is termed the dura mater. Between these layers is the delicate, cobweb-like arachnoid membrane. Inflammation of these three protective layers is called meningitis. CSF is formed by the filtration of plasma from blood in the choroid plexuses, networks of capillaries that project from the pia mater into the lateral ventricles and into the roofs of the third and fourth ventricles. After flowing through the ventricular system, the fluid circulates in the subarachnoid space (between the pia mater and the arachnoid) around the brain and spinal cord before being absorbed into venous blood through arachnoid villi. Obstruction of CSF circulation results in hydrocephalus. Meningitis is an acute inflammation of the pia mater and arachnoid, two of the membranes covering the brain and spinal cord. Infecting organisms can reach the meninges from a middle ear, the upper respiratory tract, or a frontal sinus infection, or they can be spread through the bloodstream (hematogenously) from an infection in the lungs or other site. Bacterial meningitis (pyogenic) is most commonly caused by Haemophilus influenzae in neonates and young children, and by meningococci and pneumococci in adolescents and adults. Viral meningitis may be caused by mumps, poliovirus, and occasionally herpes simplex. A chronic form of meningitis can be caused by tuberculous infection. Bacterial meningitis is the most common form. The bacteria release toxins that destroy the meningeal cells, thus stimulating immune and inflammatory reactions. Although the meninges initially demonstrate vascular congestion, edema, and minute hemorrhages, the underlying brain remains intact. MRI and CT scans are normal during most acute episodes of meningitis and remain normal if appropriate therapy is promptly instituted. If the infection extends to involve the cortex of the brain and the ependymal lining of the ventricles, contrast studies may show characteristic meningeal enhancement in the basal cisterns, interhemispheric fissure, and choroid plexus (Figure 8-4). Diffuse brain swelling may symmetrically compress the lateral and third ventricles. MRI and CT are also of value in the early detection of such complications of acute meningitis as arterial or venous vasculitis or thrombosis with infarction, hydrocephalus caused by adhesions or thickening of the arachnoid at the base of the brain, subdural effusion or empyema, and brain abscess. A spinal tap is necessary to determine the cause of meningitis. Computed tomography (CT) is the modality of choice to rule out contraindications to lumbar puncture (cerebral hemorrhage or increased ventricular pressure). The earliest sign of brain abscess on MRI or CT is an area of abnormal density with poorly defined borders and a mass effect reflecting vascular congestion and edema. Further progression of the inflammatory process leads to cerebral softening, which may undergo necrosis and liquefaction, resulting in a true abscess. MRI is considered superior for demonstrating a brain abscess, although CT can be employed when MRI is unavailable. On T1-weighted MR images, an abscess appears as a hypointense mass with an isointense capsule surrounded by low–signal intensity edema (Figure 8-5A). Both the mass and the edema are hyperintense on proton density and T2-weighted images (Figure 8-5B). After the intravenous administration of contrast material, an oval or circular peripheral ring of contrast enhancement outlines the abscess capsule. Although the wall is usually thin and of uniform thickness, an irregularly thick wall, resulting from the formation of granulation tissue, may mimic a malignant glioma. Diffusion MRI can distinguish necrotic tumors from abscesses by demonstrating a reduced diffusion coefficient. Multiple abscesses indicate the possibility of septic emboli from a systemic infection (Figure 8-6). MRI is the procedure of choice in evaluating the patient with suspected subdural empyema. Unlike CT, MRI is free from bony artifacts adjacent to the inner table of the skull. In addition, signal characteristics may permit differentiation between benign effusions and infected empyemas. Noncontrast scans demonstrate a crescentic or lentiform (lenslike), extraaxial fluid collection (representing pus) adjacent to the inner border of the skull or the falx (Figure 8-7). There is compression and displacement of the ipsilateral ventricular structures. After the intravenous administration of contrast material, a narrow zone of enhancement of relatively uniform thickness separates the extracerebral collection from the brain surface. MRI can also demonstrate involvement of the adjacent parenchyma by means of retrograde thrombophlebitis with resultant infarction or abscess formation, both of which are signs associated with a poor prognosis. Epidural empyema (Figure 8-8) is almost invariably associated with osteomyelitis in a cranial bone originating from an infection in the ear or paranasal sinuses. The infectious process is localized outside the dural membrane and beneath the inner table of the skull. The frontal region is most frequently affected because of its close relationship to the frontal sinuses and the ease with which the dura can be stripped from the bone. Acute osteomyelitis first appears radiographically as multiple small, poorly defined areas of lucency (Figure 8-9). Over the next several weeks, the lucencies enlarge and coalesce centrally with an expanding perimeter of small satellite foci. As the infection becomes more chronic (especially with syphilis, tuberculosis, or fungal infections), attempts at bone regeneration produce multiple areas of poorly defined reactive sclerosis. The clinical presentation and radiographic appearance depend on the location of the tumor and the site of the subsequent mass effect. MRI is generally considered the most sensitive technique for detecting most suspected brain tumors. In general, both the tumor and its surrounding edema demonstrate high signal intensity on T2-weighted images. After the intravenous injection of contrast material, the enhancing tumor can usually be distinguished from nonenhancing edema on T1-weighted images (Figure 8-10). In addition to its exquisite sensitivity in detecting pathologic alteration of normal tissue constituents, MRI provides excellent delineation of tumor extent and can show associated abnormalities, such as hydrocephalus. This modality is of special value in imaging neoplasms of the brainstem and posterior fossa, which may be poorly demonstrated on CT due to bone artifact. CT with contrast enhancement is an excellent examination for evaluating a patient with suspected brain tumor. It is of special value for detecting punctate or larger calcification that cannot be shown by MRI. Although skull radiographs were used in the past to demonstrate tumoral calcification, bone erosion, and displacement of the calcified pineal gland, plain images are no longer indicated because this information can be more effectively obtained on CT scans. Before the advent of CT, cerebral arteriography was used to demonstrate evidence of brain tumors, such as mass effect, contralateral displacement of midline arteries and veins, abnormal vessels with tumor staining, and early venous filling. At present, the major use of arteriography is for precise delineation of the arterial and venous anatomy. This delineation provides a surgical map before operative therapy and for evaluation of those cases in which a vascular anomaly is a strong consideration in the differential diagnosis of a tumor. Radionuclide brain scans have a relatively high rate of detection of cerebral tumors but are far less specific than CT or MRI. Positron emission tomography (PET) scans demonstrate metabolic activity and specific location of a lesion for presurgical planning (Figure 8-11). Gliomas, the most common primary malignant brain tumors, consist of glial cells (supporting connective tissues in the CNS) that still have the ability to multiply. They spread by direct extension and can cross from one cerebral hemisphere to the other through connecting white matter tracts, such as the corpus callosum. Gliomas have a peak incidence in middle adult life and are infrequent in persons less than 30 years of age. Glioblastomas are highly malignant lesions that are predominantly cerebral, although similar tumors may occur in the brainstem, cerebellum, or spinal cord. Astrocytomas (70% of all gliomas) are slow-growing tumors that have an infiltrative character and can form large cavities or pseudocysts. Favored sites are the cerebrum, cerebellum, thalamus, optic chiasm, and pons. Less frequent types of gliomas are ependymoma, medulloblastoma, and oligodendrocytoma. Ependymomas most commonly arise from the walls of the fourth ventricle, especially in children, and usually from the lateral ventricles in adults. Medulloblastomas are rapidly growing tumors, disseminating throughout the spinal fluid, which develop in the posterior portion of the vermis in children and rarely in the cerebellar hemisphere in adults. The tumor tends to spread through the subarachnoid space, with metastatic deposits occurring anywhere within the brain or spinal column. Oligodendrocytomas are slow-growing lesions that usually arise in the cerebrum and have a tendency to calcify. On MR images (Figure 8-12), gliomas typically appear as masses of high signal intensity on T2-weighted images. They may be of low intensity or isointense on T1-weighted sequences. MR spectroscopy has a typical spectral pattern with a strongly increased choline peak, which indicates myelin or the breakdown of myelin (the chemical structure that goes into making white matter). In MR spectroscopy, a highly elevated choline level, a drastically lower level of N-acetylaspartate (a neuronal marker), and a drastically lower creatine/phosphocreatine ratio confirm an infiltrating glioma (Figure 8-13). Ependymomas, often partially calcified and cystic, have a heterogeneous signal intensity and show enhancement. On noncontrast CT scans, gliomas are most commonly seen as single, nonhomogeneous masses. Low-grade astrocytomas tend to be low-density lesions showing little or no enhancement (Figure 8-14); glioblastomas most frequently contain areas of both increased and decreased density, although a broad spectrum of CT appearances can occur. Edema is often seen in the adjacent subcortical white matter. After the intravenous injection of contrast material, virtually all gliomas show enhancement, with the most malignant lesions tending to be enhanced to the greatest degree (see Figure 8-10). In MR spectroscopy, an elevated choline/creatine ratio suggests a malignant neoplasm. The most common pattern is an irregular ring of contrast enhancement, representing solid vascularized tumor, surrounding a central low-density area of necrosis. Contrast enhancement also can appear as patches of increased density distributed irregularly throughout a low-density lesion or as rounded nodules of increased density within the mass. Because meningiomas tend to be isointense with brain on both T1- and T2-weighted images, anatomic distortion is the key to the MRI diagnosis (Figure 8-15). A thin rim of low intensity, consisting of a CSF cleft, the vascular rim, or dura, may separate the tumor from the adjacent brain. Calcification within a meningioma may produce nonuniform signal or focal signal void. Surrounding edema may make the lesion easier to identify. Just as the detection of meningiomas by CT is facilitated by the use of iodinated contrast material, paramagnetic contrast agents can enhance the detection of meningiomas on MRI. MR spectroscopy has a typical spectral pattern with a strongly increased choline peak; creatine is a marker of energy metabolism. Alanine is another specific amino acid marker producing a unique peak in meningiomas. CT typically shows a meningioma as a rounded, sharply delineated, isodense (25%) or hyperdense (75%) tumor abutting a dural surface. Calcification often is seen within the mass on noncontrast scans. After the intravenous injection of contrast material, there is intense homogeneous enhancement, which reflects the highly vascular nature of the tumor (Figure 8-16). Pronounced dilatation of meningeal and diploic vessels, which provide part of the blood supply to the tumor, may produce prominent grooves in the calvaria on plain images of the skull. Calvarial hyperostosis (increased density) may develop because of invasion of the bone by tumor cells that stimulate osteoblastic activity. Dense calcification or granular psammomatous deposits may be seen within the tumor (Figure 8-17). Spinal meningiomas are best demonstrated on T1- or T2-weighted MR images using gadolinium enhancement as a homogeneous intense lesion. CT myelography demonstrates the location of the mass, which is usually intradural extramedullary (Figure 8-18).
Nervous System
Physiology of the nervous system
Infections of the central nervous system
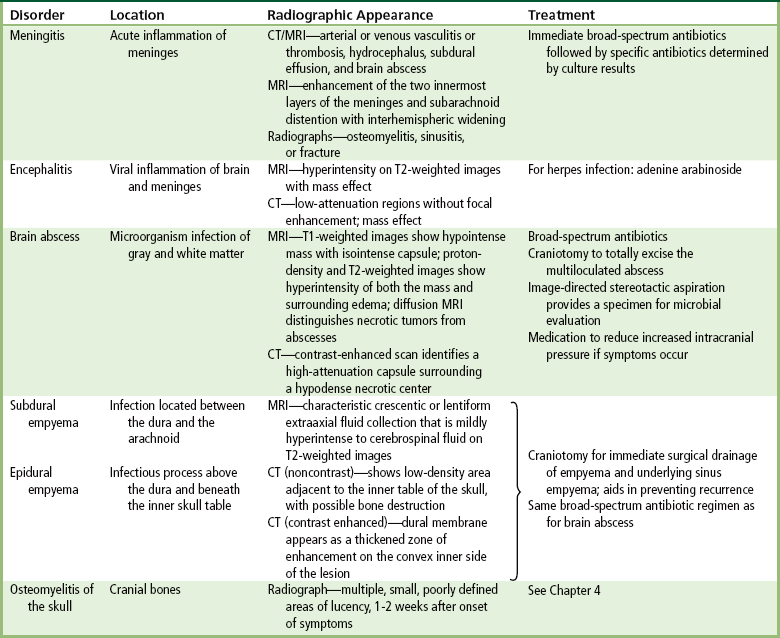
Meningitis
Radiographic Appearance
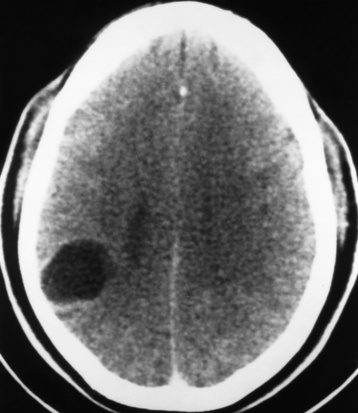
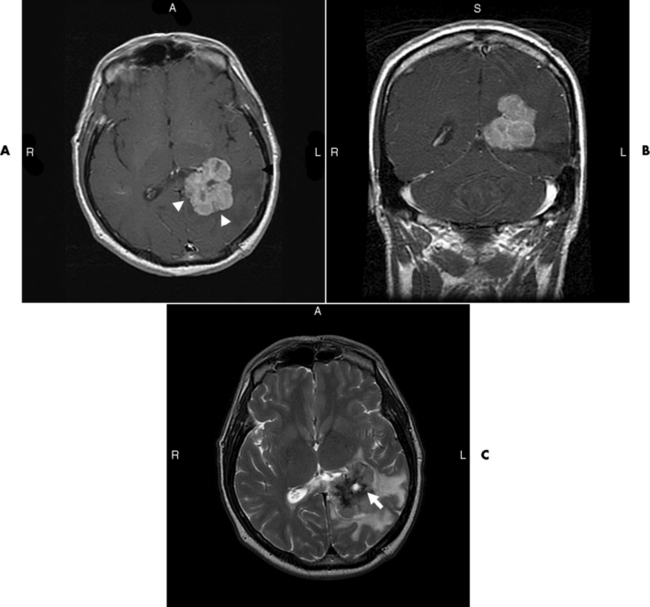
Brain Abscess
Radiographic Appearance
Subdural Empyema
Radiographic Appearance
Epidural Empyema
Osteomyelitis of the Skull
Radiographic Appearance
Tumors of the central nervous system
Radiographic Appearance
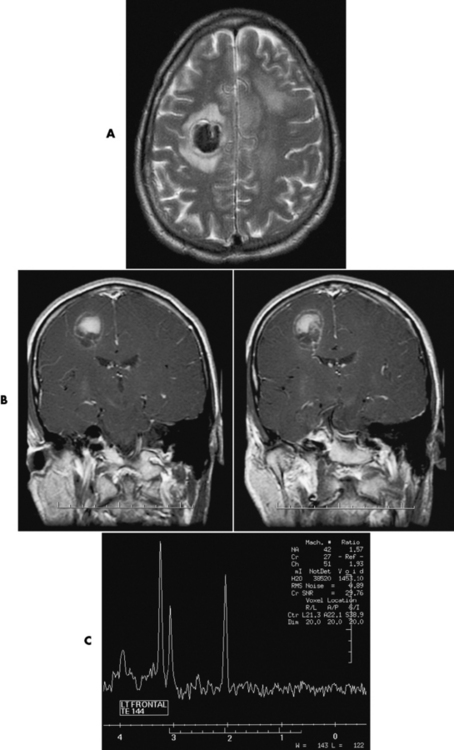
Glioma
Radiographic Appearance
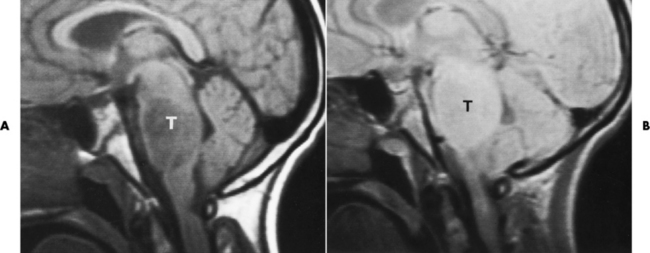
Meningioma
Radiographic Appearance
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nervous System