NEURONS
GLIAL CELLS & NEURONAL ACTIVITY
NEURAL PLASTICITY & REGENERATION
The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting cells called glial cells. Each neuron has hundreds of interconnections with other neurons, forming a very complex system for processing information and generating responses.
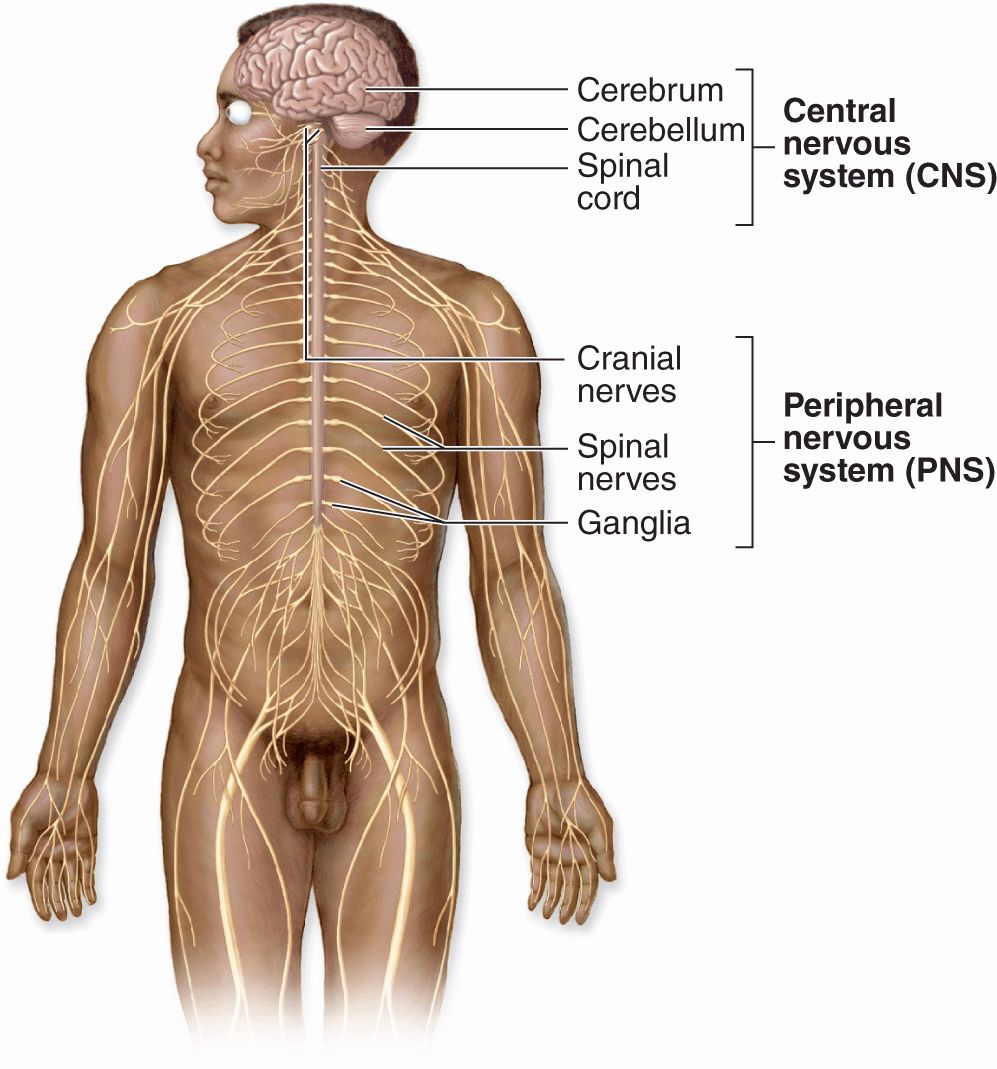
Nerve tissue is distributed throughout the body as an integrated communications network. Anatomically, the general organization of the nervous system (Figure 9–1) has two major divisions:
FIGURE 9–1 The general organization of the nervous system.
 Central nervous system (CNS), consisting of the brain and spinal cord
Central nervous system (CNS), consisting of the brain and spinal cord
 Peripheral nervous system (PNS), composed of the cranial, spinal, and peripheral nerves conducting impulses to and from the CNS (sensory and motor nerves, respectively) and ganglia that are small groups of nerve cells outside the CNS.
Peripheral nervous system (PNS), composed of the cranial, spinal, and peripheral nerves conducting impulses to and from the CNS (sensory and motor nerves, respectively) and ganglia that are small groups of nerve cells outside the CNS.
Cells in both central and peripheral nerve tissue are of two kinds: nerve cells, or neurons, which usually show numerous long processes; and various glial cells (Gr. glia, glue), which have short processes, support and protect neurons, and participate in many neural activities, neural nutrition, and defense of cells in the CNS.
Neurons respond to environmental changes (stimuli) by altering the ionic gradient that exists across their plasma membranes. All cells maintain such a gradient, also called an electrical potential, but cells that can rapidly change this potential in response to stimuli (eg, neurons, muscle cells, some gland cells) are said to be excitable or irritable. Neurons react promptly to stimuli with a reversal of the ionic gradient (membrane depolarization) that generally spreads from the place that received the stimulus and is propagated across the neuron’s entire plasma membrane. This propagation, called the action potential, the depolarization wave, or the nerve impulse, is capable of traveling long distances along neuronal processes, transmitting such signals to other neurons, muscles, and glands.
By collecting, analyzing, and integrating information in such signals, the nervous system continuously stabilizes the intrinsic conditions of the body (eg, blood pressure, O2 and CO2 content, pH, blood glucose levels, and hormone levels) within normal ranges and maintains behavioral patterns (eg, feeding, reproduction, defense, interaction with other living creatures).
DEVELOPMENT OF NERVE TISSUE
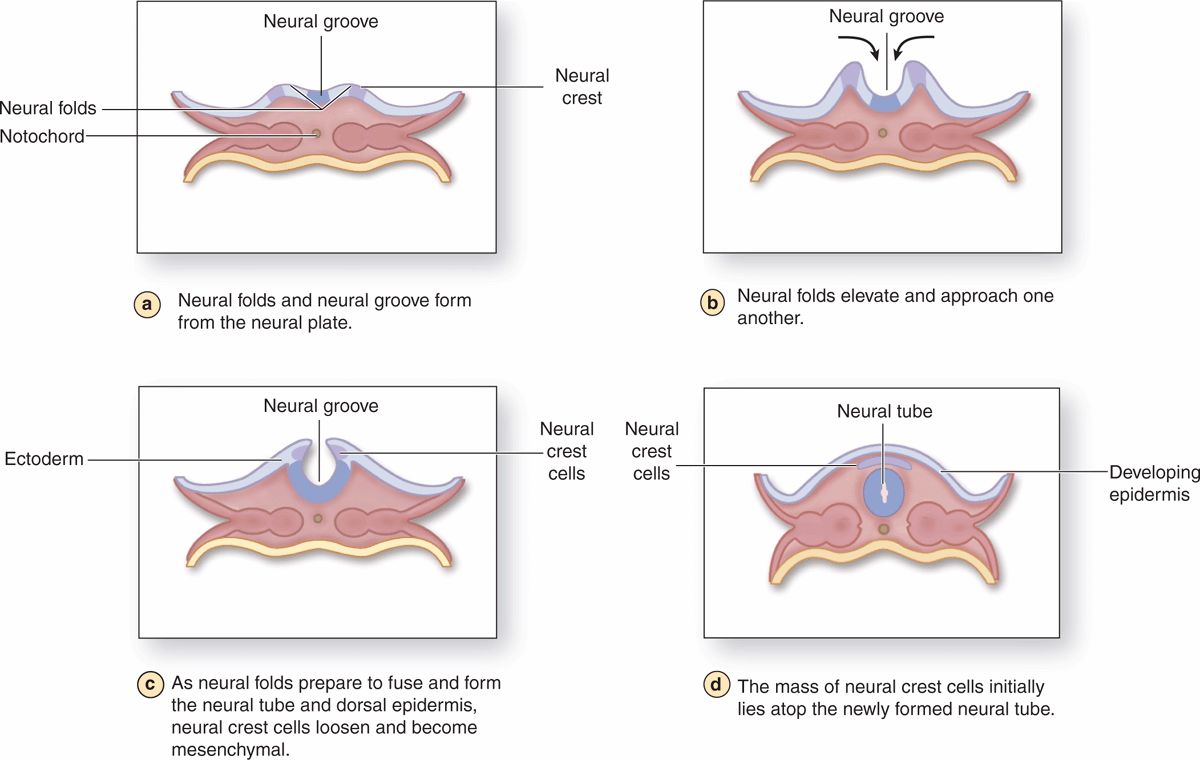
The nervous system develops from the outermost of the three early embryonic layers, the ectoderm, beginning in the third week of development (Figure 9–2). With signals from the underlying axial structure, the notochord, ectoderm on the mid-dorsal side of the embryo thickens to form the epithelial neural plate. The lateral sides of this plate fold upward, bend and grow toward each other medially, and within a few days fuse to form the neural tube. Cells of this tube give rise to the entire CNS, including neurons and most glial cells.
FIGURE 9-2 Neurulation in the early embryo.
As the folds fuse and the neural tube separates from the now overlying surface ectoderm that will form epidermis, a large population of developmentally important cells, the neural crest, separates from the neuroepithelium and becomes mesenchymal. Neural crest cells migrate extensively and differentiate as all the cells of the PNS, as well as a number of other nonneuronal cell types.
NEURONS
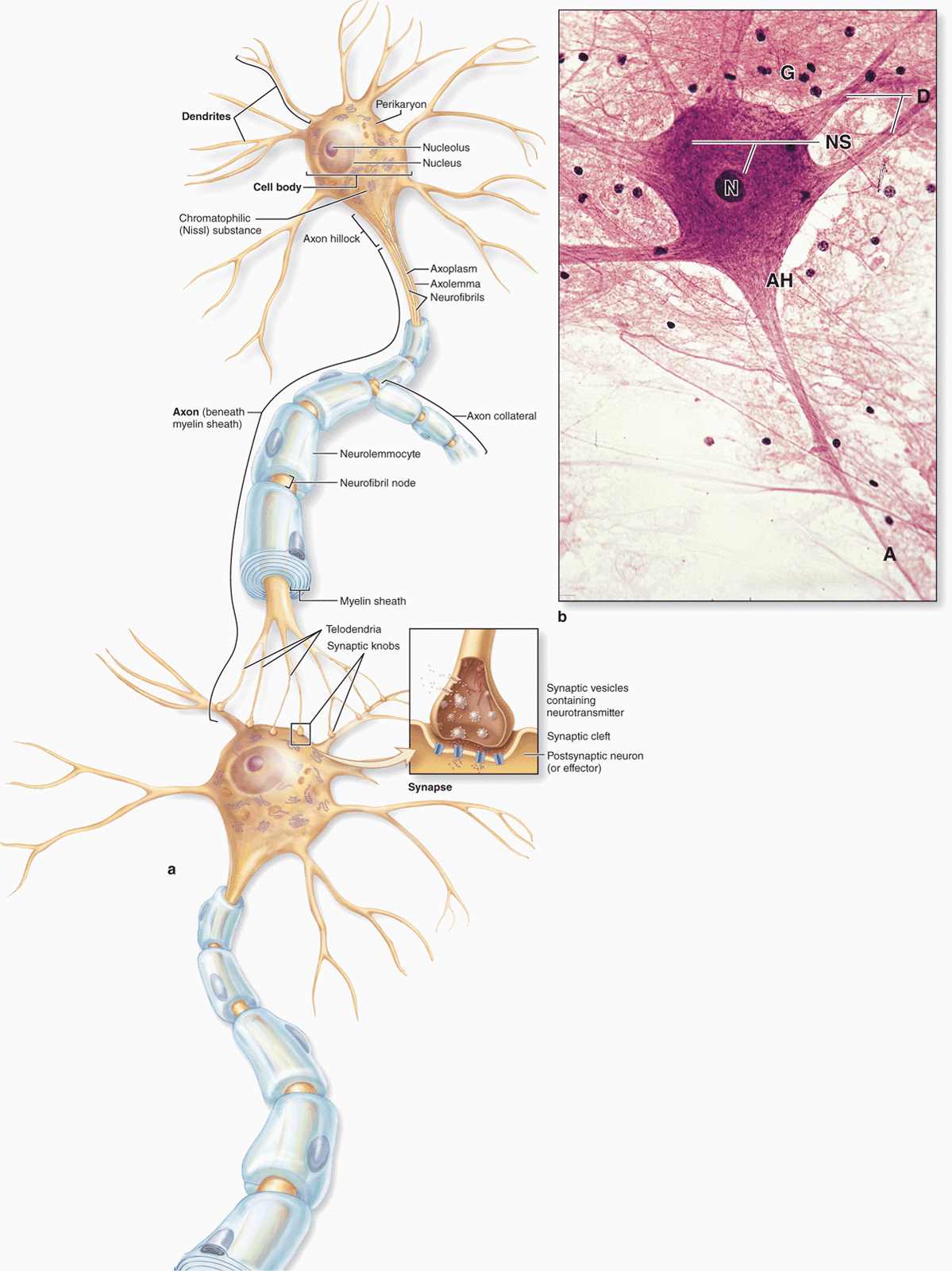
The functional unit in both the CNS and PNS is the neuron or nerve cell. Some neuronal components have special names, such as “neurolemma” for the cell membrane. Most neurons consist of three main parts (Figure 9–3):
FIGURE 9–3 Structures of neuron.
 The cell body, or perikaryon, which contains the nucleus and most of the cell’s organelles and serves as the synthetic or trophic center for the entire neuron.
The cell body, or perikaryon, which contains the nucleus and most of the cell’s organelles and serves as the synthetic or trophic center for the entire neuron.
 The dendrites, which are the numerous elongated processes extending from the perikaryon and specialized to receive stimuli from other neurons at unique sites called synapses.
The dendrites, which are the numerous elongated processes extending from the perikaryon and specialized to receive stimuli from other neurons at unique sites called synapses.
 The axon (Gr. axon, axis), which is a single long process ending at synapses specialized to generate and conduct nerve impulses to other cells (nerve, muscle, and gland cells). Axons may also receive information from other neurons, information that mainly modifies the transmission of action potentials to those neurons.
The axon (Gr. axon, axis), which is a single long process ending at synapses specialized to generate and conduct nerve impulses to other cells (nerve, muscle, and gland cells). Axons may also receive information from other neurons, information that mainly modifies the transmission of action potentials to those neurons.
Neurons and their processes are extremely variable in size and shape. Cell bodies can be very large, measuring up to 150 μm in diameter. Other neurons, such as the cerebellar granule cells, are among the body’s smallest cells.
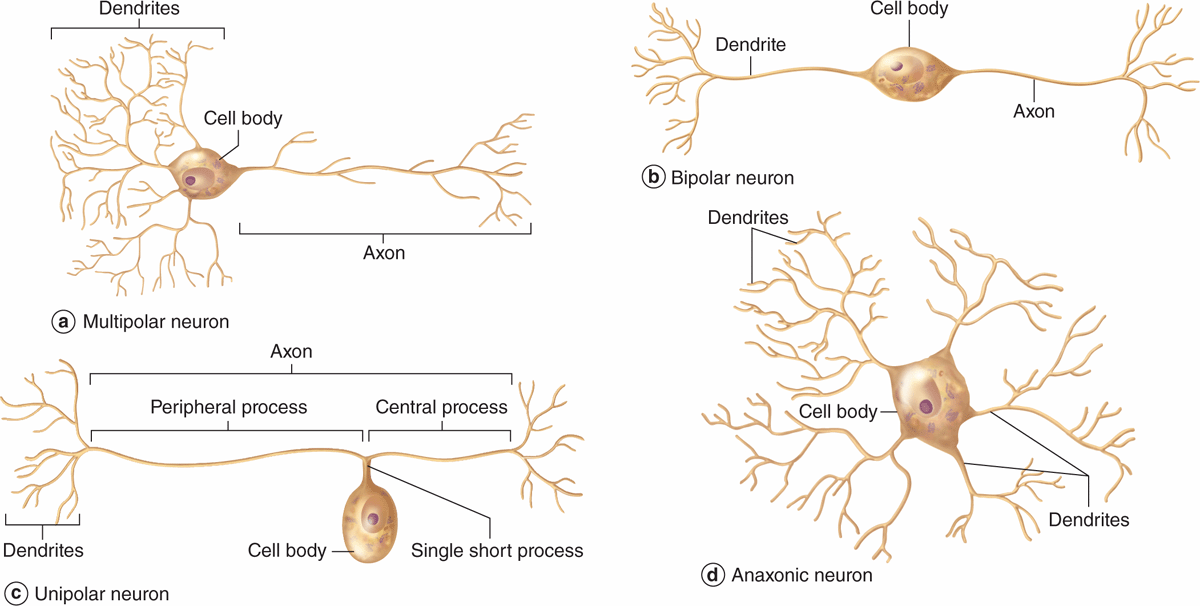
Neurons can be classified according to the number of processes extending from the cell body (Figure 9–4):
FIGURE 9–4 Structural classes of neurons.
 Multipolar neurons, which have one axon and two or more dendrites
Multipolar neurons, which have one axon and two or more dendrites
 Bipolar neurons, with one dendrite and one axon
Bipolar neurons, with one dendrite and one axon
 Unipolar or pseudounipolar neurons, which have a single process that bifurcates close to the perikaryon, with the longer branch extending to a peripheral ending and the other toward the CNS.
Unipolar or pseudounipolar neurons, which have a single process that bifurcates close to the perikaryon, with the longer branch extending to a peripheral ending and the other toward the CNS.
 Anaxonic neurons, with many dendrites but no true axon, do not produce action potentials, but regulate electrical changes of adjacent neurons.
Anaxonic neurons, with many dendrites but no true axon, do not produce action potentials, but regulate electrical changes of adjacent neurons.
Most neurons are multipolar. Bipolar neurons are found in the retina, olfactory mucosa, and the (inner ear) cochlear and vestibular ganglia, where they serve the senses of sight, smell, and balance, respectively. Pseudounipolar neurons are found in the spinal ganglia (the sensory ganglia found with the spinal nerves) and in most cranial ganglia. Because the fine processes emerging from perikarya are seldom seen in sections of nervous tissue, it is difficult to classify neurons structurally by microscopic inspection.
Nervous components can also be subdivided functionally (Figure 9–1). Sensory neurons are afferent and receive stimuli from the receptors throughout the body. Motor neurons are efferent, sending impulses to effector organs such as muscle fibers and glands. Somatic motor nerves are under voluntary control and typically innervate most skeletal muscle; autonomic motor nerves control the “involuntary” activities of glands, cardiac muscle, and most smooth muscle.
Interneurons establish relationships among other neurons, forming complex functional networks or circuits (as in the CNS and retina). Interneurons are generally multipolar or anaxonic and are estimated to include 99% of the neurons in the human CNS.
In the CNS most neuronal perikarya occur in the gray matter, with axons concentrated in the white matter. These terms refer to the general appearance of unstained CNS tissue caused in part by the different densities of nerve cell bodies. In the PNS cell bodies are found in ganglia and in some sensory regions, such as the olfactory mucosa, and axons are bundled in nerves.
Cell Body (Perikaryon)
The cell body is the neuronal region that contains the nucleus and surrounding cytoplasm, exclusive of the cell processes (Figure 9–3). It acts as a trophic center, producing cytoplasm for movement into the processes, although most cell bodies also receive a great number of nerve endings conveying excitatory or inhibitory stimuli generated in other nerve cells. Most nerve cells have a generally spherical, unusually large, euchromatic (pale-staining) nucleus with a prominent nucleolus. The chromatin is finely dispersed, reflecting the intense synthetic activity of these cells.
Cytoplasm of perikarya often contains a highly developed RER with many parallel cisternae and neighboring regions with numerous polyribosomes, indicating active production of both cytoskeletal proteins and proteins for transport and secretion. Histologically these regions with concentrated RER and other polysomes appear as clumps of basophilic material called chromatophilic substance (or Nissl substance, Nissl bodies) (Figure 9–3). The amount of this basophilic material varies with the type and functional state of the neuron and is particularly abundant in large nerve cells such as motor neurons (Figure 9–3b). The Golgi apparatus is located only in the cell body, but mitochondria can be found throughout the cell and are usually abundant in the axon terminals.
Intermediate filaments are abundant both in perikarya and processes and in this cell are often called neurofilaments. Neurofilaments become cross-linked with certain fixatives and, when impregnated with silver stains, they form neurofibrils visible with the light microscope. Neurons also contain microtubules identical to those found in other cells. Nerve cells occasionally contain inclusions of pigmented material, such as lipofuscin, consisting of residual bodies left from lysosomal digestion.
Dendrites
Dendrites (Gr. dendron, tree) are usually short and divided like tree branches (Figure 9–3). They are usually covered with many synapses and are the principal signal reception and processing sites on neurons. Most nerve cells have many dendrites, which increase the receptive area of the cell considerably. The arborization of dendrites makes it possible for one neuron to receive and integrate a great number of axon terminals from other nerve cells. For example, it has been estimated that up to 200,000 axonal endings make functional contact with the dendrites of a single large Purkinje cell of the cerebellum.
Unlike axons, which maintain a nearly constant diameter, dendrites become much thinner as they subdivide. The cytoplasm of the dendrite base is similar to that of the perikaryon, with cytoskeletal elements predominating in the branched regions. Most synapses impinging on neurons occur on dendritic spines, which are short blunt structures projecting at points along dendrites, visible with silver staining methods (Figure 9–5). Dendritic spines occur in vast numbers, estimated to be on the order of 1014 for cells of the human cerebral cortex, and serve as the initial processing sites for synaptic signals. The morphology of these spines depends on actin filaments and can be highly plastic; dendritic spines are of key importance in the constant changes of the neural plasticity underlying adaptation, learning, and memory.
FIGURE 9–5 Dendrites and dendritic spines.
Axons
Most neurons have only one axon, a fine cylindrical process that varies in length and diameter according to the type of neuron. Axons are usually very long processes. For example, axons of the motor neurons of the spinal cord that innervate the foot muscles may have a length of nearly 100 cm and require large cell bodies for their maintenance. Axons originate from a pyramid-shaped region of the perikaryon called the axon hillock (Figure 9–3). The plasma membrane of the axon is often called the axolemma and its contents are known as axoplasm.
Just beyond the axon hillock, at an area called the initial segment, is the site where various excitatory and inhibitory stimuli impinging on the neuron are algebraically summed, resulting in the decision to propagate—or not to propagate—a nerve impulse. The axolemma of the initial segment contains various ion channels important in generating the action potential.
In contrast to dendrites, the typical axon is much longer, has a constant diameter, and branches less profusely. As shown in Figure 9–3, however, the distal end of an axon forms a terminal arborization, and axons of interneurons and some motor neurons have branches called collaterals that end at synapses influencing the activity of many other neurons. Each branch ends with a dilation called a terminal bouton (Fr. bouton, button) that contacts another neuron or non-nerve cell at a synapse to initiate an impulse in that cell. Axoplasm contains mitochondria, microtubules, neurofilaments, and some cisternae of smooth ER, but essentially no polyribosomes or RER, emphasizing its dependence on the perikaryon for maintenance. If an axon is severed, its peripheral part quickly degenerates.
There is a lively bidirectional transport of small and large molecules along the axon. Organelles and macromolecules synthesized in the cell body move by anterograde transport along the axon from the perikaryon to the synaptic terminals. Retrograde transport in the opposite direction carries certain other macromolecules, such as material taken up by endocytosis (including viruses and toxins), from the periphery to the cell body. Retrograde transport can be used to study the pathways of neurons: if peroxidase or another marker is injected into regions with axon terminals, its distribution along the entire axon after a period of time can be determined histochemically.
Axonal transport in both directions uses motor proteins on microtubules, as discussed in Chapter 2. Kinesin, a micro-tubule-activated ATPase, mediates anterograde vesicular transport, and the similar ATPase called cytoplasmic dynein provides retrograde transport. Anterograde and retrograde transports both occur fairly rapidly, at rates of 50-400 mm/d. A much slower anterograde stream (only a few millimeters per day) involves movement of the axonal cytoskeleton itself. This slow axonal transport system corresponds roughly to the rate of axon growth.
Nerve Impulses
A nerve impulse, or action potential, travels along an axon like a spark moves along an explosive’s fuse. It is an electrochemical process initiated at the axon hillock when other impulses received at the cell body or dendrites meet a certain threshold. The action potential is propagated along the axon as a wave of membrane depolarization produced by voltage-gated Na+ and K+ channels in the axolemma that allow diffusion of these ions into and out of the axoplasm. The extracellular compartment around all regions of the neuron is a very thin zone immediately outside the cell that is formed by enclosing glial cells that regulate its ionic contents.
In unstimulated neurons, ATP-dependent Na-K pumps and other membrane proteins maintain an axoplasmic Na+ concentration only one-tenth that outside the cell and a K+ level many times greater than the extracellular concentration. This produces a potential electrical difference across the axolemma of about -65 mV, with the inside negative to the outside. This difference is the axon’s resting potential.
When the threshold triggering an impulse is met, channels at the axon’s initial segment open and allow a very rapid influx of extracellular Na+ that makes the axoplasm positive in relation to the extracellular environment and shifts (depolarizes) the resting potential from negative to positive, to +30 mV. Immediately after the membrane depolarization, the voltage-gated Na+ channels close and those for K+ open, and this rapidly returns the membrane to its resting potential. This cycle of events occurs in less than 1 millisecond.
Depolarization stimulates adjacent portions of the axolemma to depolarize and return immediately to the resting potential, causing the nerve impulse, or wave of depolarization, to move rapidly along the axon. After a refractory period also measured in milliseconds, the neuron is ready to repeat the process and generate another action potential. Impulses arriving at the synaptic nerve endings promote the discharge of stored neurotransmitter that stimulates or inhibits action potentials in another neuron or a non-neural cell.
Synaptic Communication
Synapses (Gr. synapsis, union) are sites where nerve impulses are transmitted from one neuron to another or from neurons and other effector cells. The structure of a synapse (Figure 9–6) ensures that transmission is unidirectional. Synapses convert an electrical signal (nerve impulse) from the presynaptic cell into a chemical signal that affects the postsynaptic cell. Most synapses act by releasing neurotransmitters, which are usually small molecules that bind specific receptor proteins to either open or close ion channels or initiate second-messenger cascades. A synapse (Figure 9–6a) has the following components:
FIGURE 9-6 Major components of a synapse.
 Presynaptic axon terminal (terminal bouton) from which neurotransmitter is released by exocytosis from synaptic vesicles.
Presynaptic axon terminal (terminal bouton) from which neurotransmitter is released by exocytosis from synaptic vesicles.
 Postsynaptic cell membrane with receptors for the transmitter and ion channels or other mechanisms to initiate a new impulse.
Postsynaptic cell membrane with receptors for the transmitter and ion channels or other mechanisms to initiate a new impulse.
 A 20- to 30-nm-wide intercellular space called the synaptic cleft separating the presynaptic and postsynaptic membranes.
A 20- to 30-nm-wide intercellular space called the synaptic cleft separating the presynaptic and postsynaptic membranes.
At the presynaptic region the nerve impulse briefly opens calcium channels, promoting a Ca2+ influx that triggers neurotransmitter release by exocytosis or similar mechanisms. Released neurotransmitter molecules diffuse immediately across the synaptic cleft and bind receptors at the postsynaptic region, producing either an excitatory or an inhibitory effect at the postsynaptic membrane.
 Neurotransmitters from excitatory synapses cause postsynaptic Na+ channels to open, and the resulting influx of this ion initiates a depolarization wave in that neuron or effector cell as described previously.
Neurotransmitters from excitatory synapses cause postsynaptic Na+ channels to open, and the resulting influx of this ion initiates a depolarization wave in that neuron or effector cell as described previously.
 At inhibitory synapses neurotransmitters open Cl– or other anion channels, causing influx of anions and hyperpolarization of the postsynaptic cell, making its membrane potential more negative and more resistant to depolarization.
At inhibitory synapses neurotransmitters open Cl– or other anion channels, causing influx of anions and hyperpolarization of the postsynaptic cell, making its membrane potential more negative and more resistant to depolarization.
Interplay between excitatory and inhibitory effects on postsynaptic cells allows synapses to process neuronal input and fine-tune the reaction of the effector cell. Impulses passing from presynaptic neurons to postsynaptic cells are usually modified at the synapse by similar connections there from other neurons (see Figure 9–6b). Activity of postsynaptic neurons is determined by the summation of activity at hundreds of synapses on that cell.
Once released, neurotransmitters are removed quickly by enzymatic breakdown, diffusion, or endocytosis (recycling) mediated by specific receptors on the presynaptic membrane. Removal of neurotransmitters prevents sustained stimulation of the postsynaptic cell.
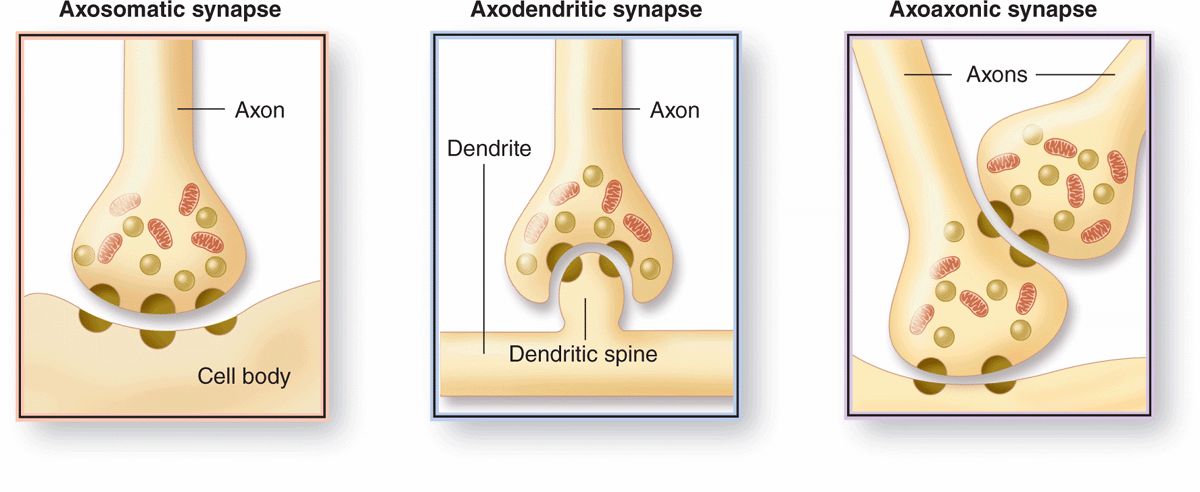
Morphologically, various types of synapses are seen between neurons (Figure 9–7). If an axon forms a synapse with a cell body, it is called an axosomatic synapse; with a dendrite, axodendritic; or with another axon, axoaxonic. Axoaxonic synapses modulate activity of the other two types. Synaptic structure cannot be resolved by light microscopy, although components such as dendritic spines may be shown by methods such as silver precipitation (see Figure 9–5).
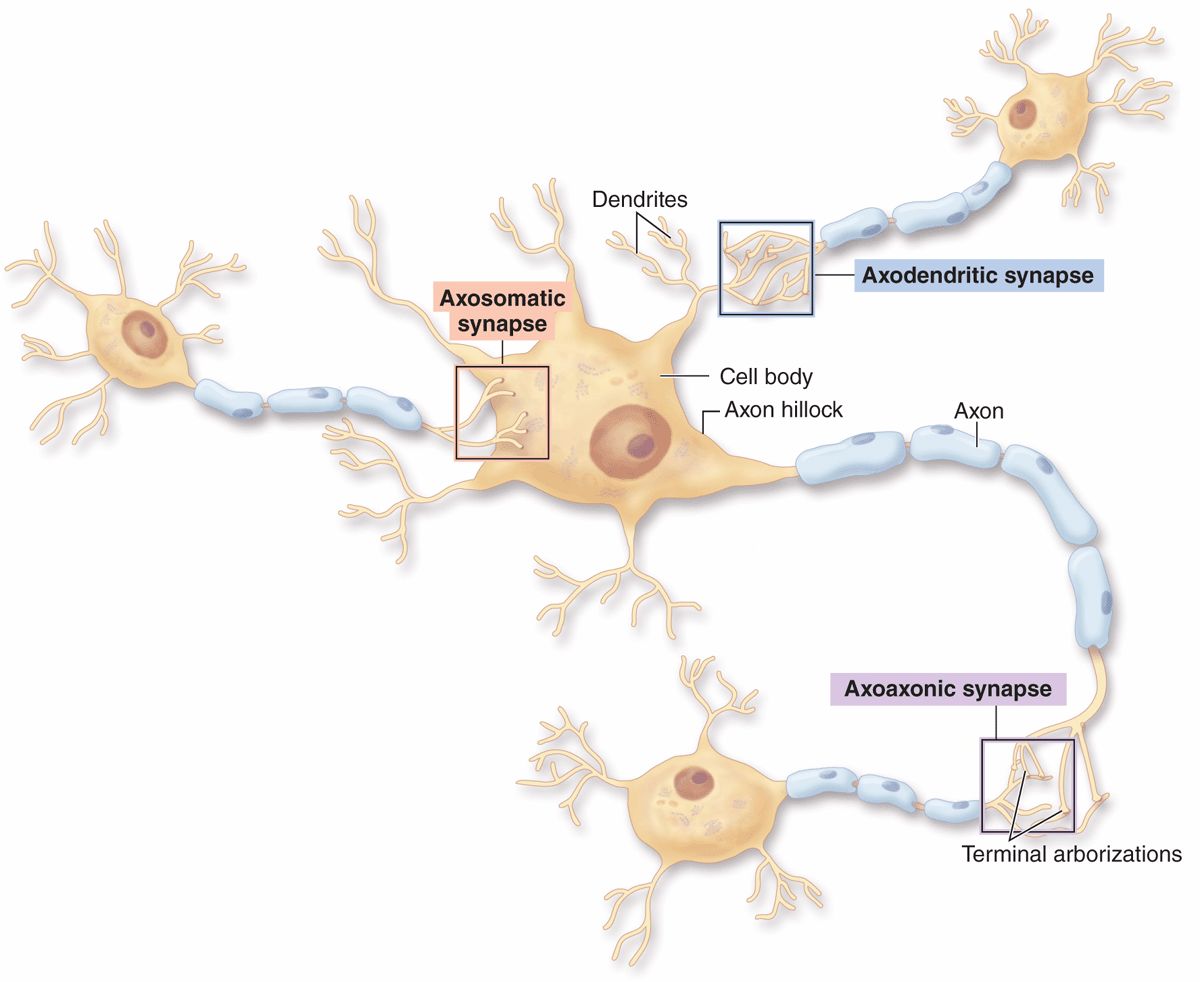
FIGURE 9–7 Types of synapses.
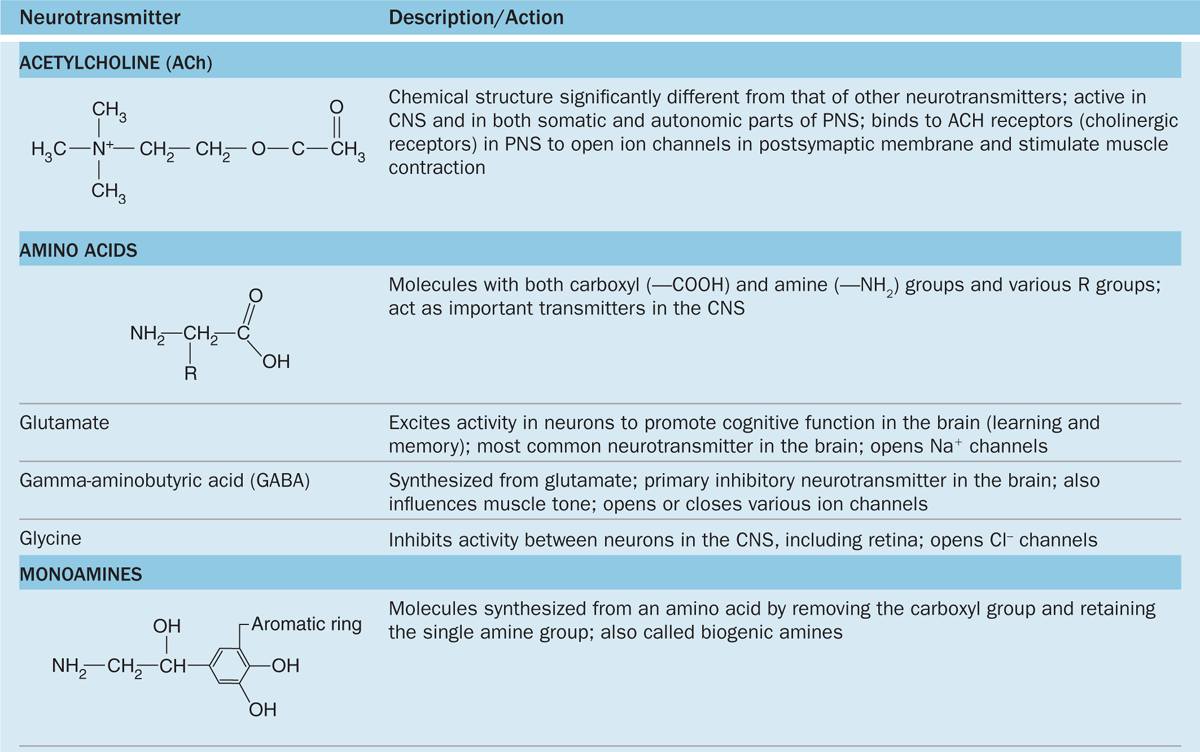
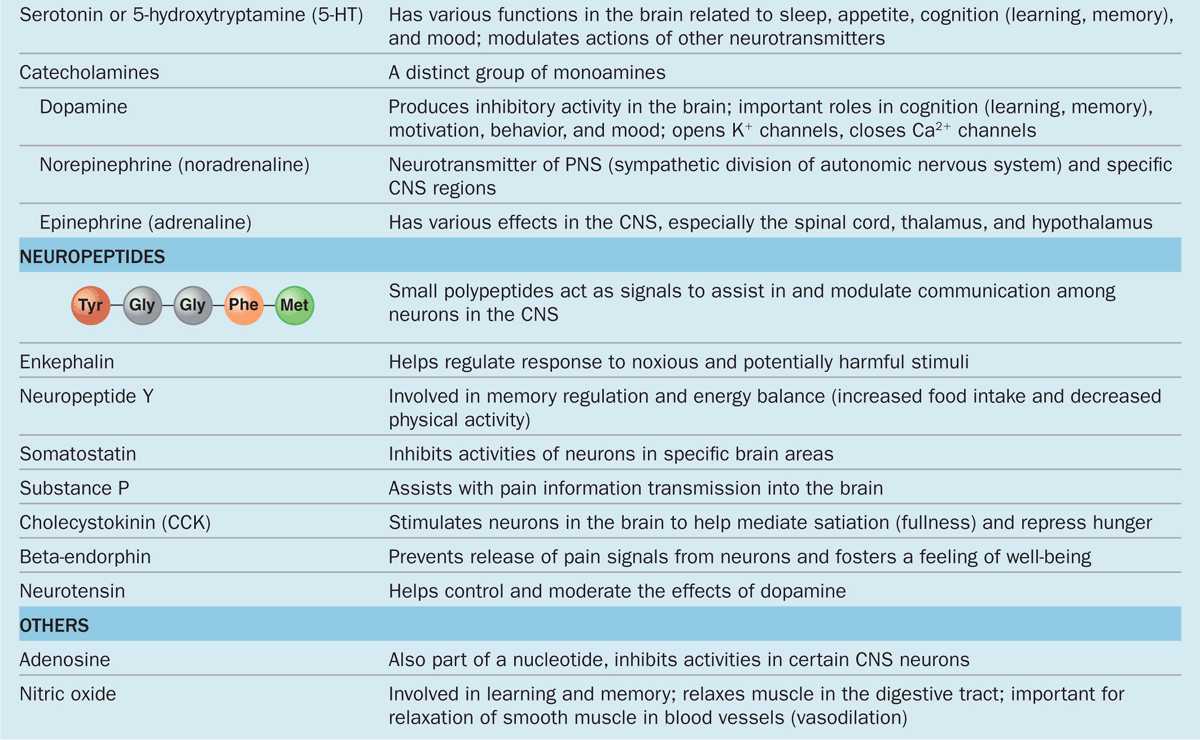
The chemical transmitter used at neuromuscular junctions is acetylcholine. Within the CNS major categories of neurotransmitters include the following:
 Catecholamines, such as epinephrine (adrenalin), norepinephrine, and dopamine;
Catecholamines, such as epinephrine (adrenalin), norepinephrine, and dopamine;
 Amino acids (often modified), such as glutamate, glycine, serotonin (5-hydroxytryptamine or 5-HT), and γ-aminobutyrate (GABA); and
Amino acids (often modified), such as glutamate, glycine, serotonin (5-hydroxytryptamine or 5-HT), and γ-aminobutyrate (GABA); and
 Small peptides, such as endorphins and substance P.
Small peptides, such as endorphins and substance P.
The actions of these and other common neurotransmitters are summarized in Table 9–1.
TABLE 9–1 Common neurotransmitters and their actions.
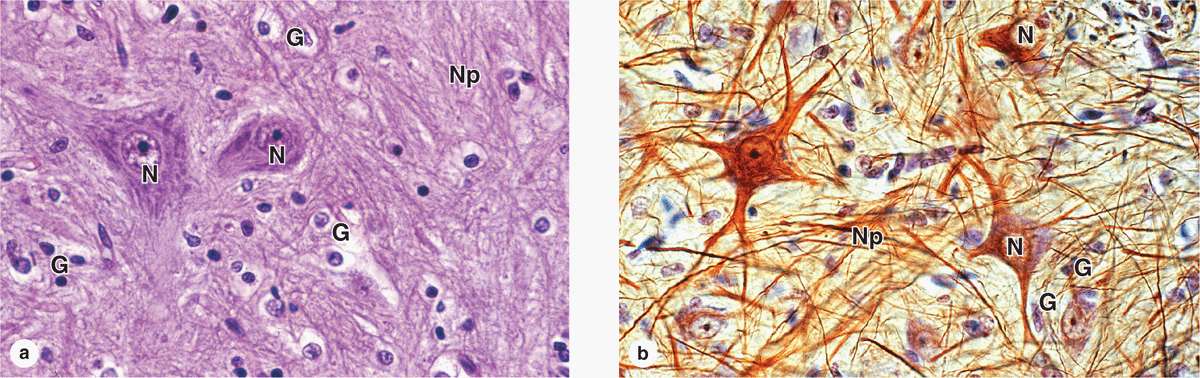
GLIAL CELLS & NEURONAL ACTIVITY
Glial cells support neuronal survival and activities, and are ten times more abundant in the mammalian brain than the neurons. Like neurons, most glial cells develop from progenitor cells of the embryonic neural plate. In the CNS glial cells surround both the neuronal cell bodies, which are often larger than glial cells, and the processes of axons and dendrites occupying the spaces between neurons. Except around the larger blood vessels, the CNS has only a very small amount of connective tissue and collagen. Glial cells substitute for cells of connective tissue in some respects, supporting neurons and creating a microenvironment immediately around those cells that is optimal for neuronal activity. The fibrous intercellular network surrounding cells of the CNS may superficially resemble collagen with light microscopy, but it is actually the network of cellular processes emerging from neurons and glial cells. Such processes are collectively called the neuropil (Figure 9–8).
FIGURE 9–8 Neurons, neuropil, and the common glial cells of the CNS.
 MEDICAL APPLICATION
MEDICAL APPLICATION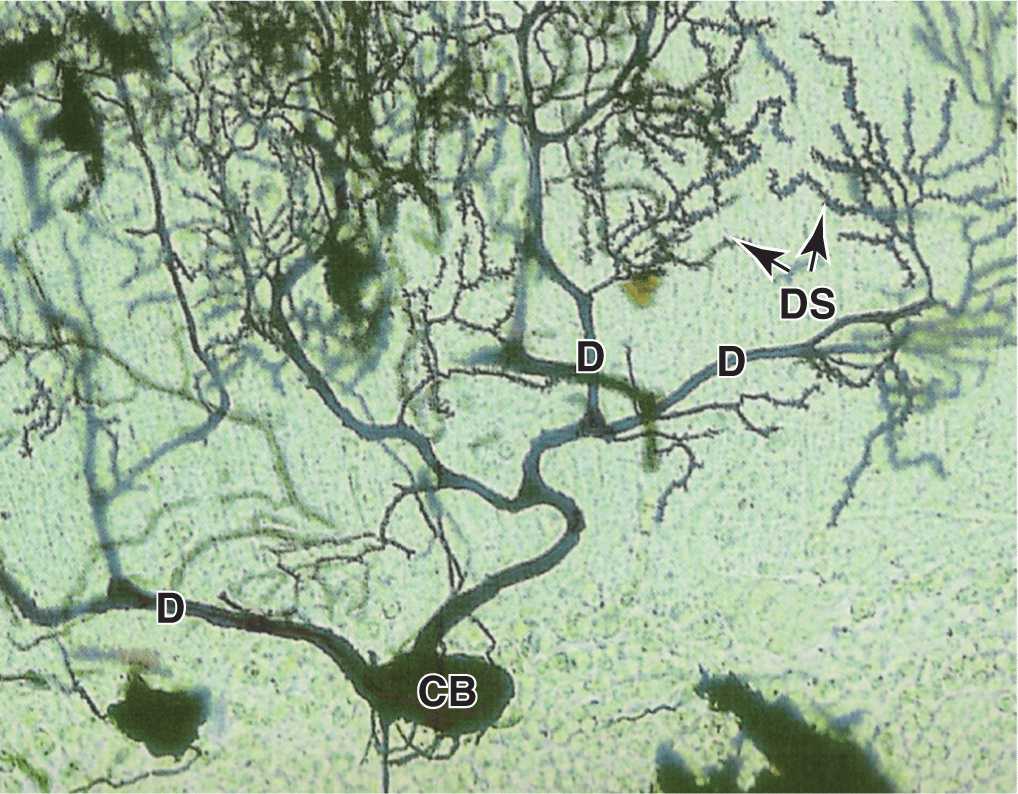
 MEDICAL APPLICATION
MEDICAL APPLICATION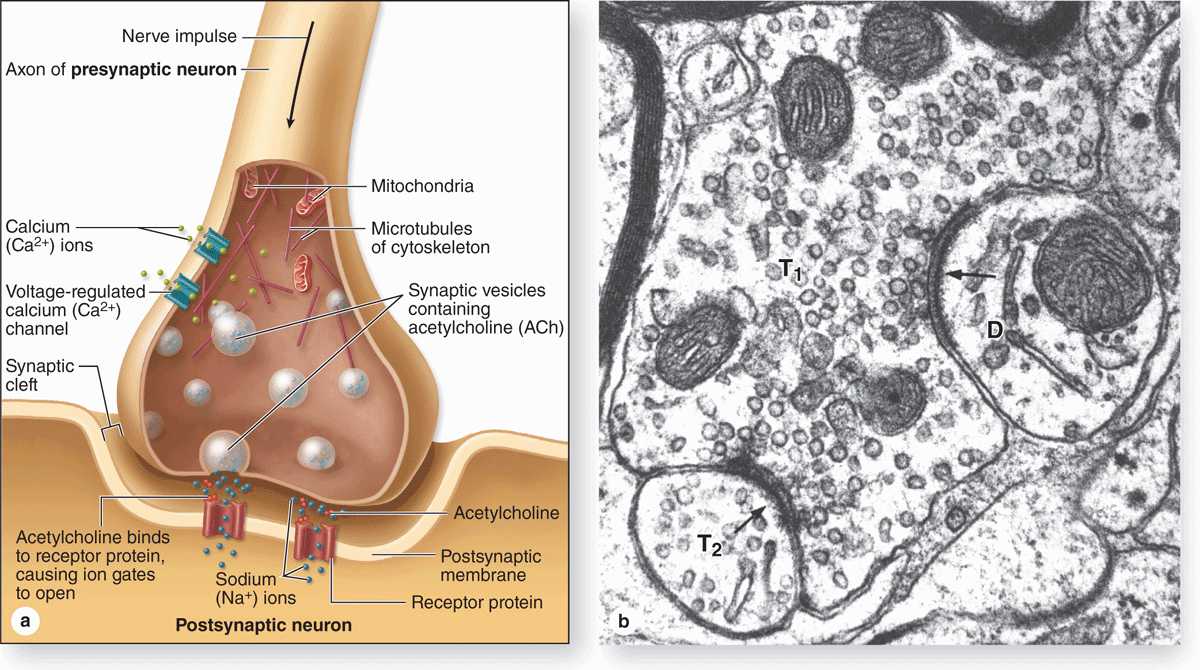
 MEDICAL APPLICATION
MEDICAL APPLICATION