I. NORMAL PERIPHERAL NERVE AND METHODS OF ANALYSIS. The sural nerve, a cutaneous sensory nerve to the lateral foot, is typically biopsied without stretching or use of intraneural anesthetic. The nerve is divided into a portion for fixation in formalin for paraffin embedding, and a portion for fixation in glutaraldehyde for plastic sections and possible electron microscopy. The entire nerve is typically used rather than dissected into individual fascicles. The portion (˜1 cm) of the nerve fixed in formalin should be cut into 3 mm segments and examined in crosssection with paraffin-embedded H&E sections, thioflavin-S histochemistry, and possible immunohistochemistry.
An H&E-stained cross section of the sural nerve (e-Fig. 42.1)* contains 6 to 12 fascicles, each surrounded by flattened perineurial cells. Outside the perineurium is the epineurium, containing connective tissue and an anastomotic vascular network. Often individual myelinated axons can be seen in H&E-stained material (e-Fig. 42.2). The endoneurium (the space inside of the perineurial cell layer and outside the axon/Schwann cell units) contains fluid, collagen, capillaries, venules, fibroblasts, macrophages/monocytes, and scattered mast cells. Immunohistologic localization is useful for the demonstration of neurofilaments (a rough measure of axon number, e-Fig. 42.3), amyloid, immunoglobulins, subtypes of inflammatory cells, and growth factors and their receptors.
Plastic-embedded sections of 1 µm thickness (e-Fig. 42.4) provide a wealth of information. Myelinated axons range in diameter from 2 to 18 µm and are coarsely separated into small (mean of 4 µm) and large (˜12 µm) myelinated axon populations (e-Figs. 42.5 and 42.6) with myelin thickness related directly to axonal diameter. The patterns of nerve damage visible in plastic sections may be characteristic (although rarely pathognomonic) of certain disease entities or pathogenetic processes. Qualitative information is provided on the degree of myelinated axon loss, distribution of axon loss, presence of active axonal degeneration (AD) or demyelination, identification of regenerative clusters of axons, swollen axons, onion-bulb formation, the nature of cellular infiltrates, or amyloid deposition. Groups of unmyelinated axon populations are detectable in plastic sections (e-Fig. 42.6).
Ultrastructure provides additional detail and is the only definitive method to evaluate unmyelinated axons (e-Fig. 42.7), which are three- to fourfold more numerous than myelinated axons in the sural nerve and typically are <2 µm in diameter.
If necessary, lengths of individual lightly fixed, osmicated myelinated axons can be dissected or “teased” out of a fascicle with pins. Each myelin internode, maintained by a single Schwann cell, ranges from 0.2 to 1.8 mm in length, increasing
linearly with axon diameter. Ongoing activity or residua of past episodes of demyelination or AD are readily identified.
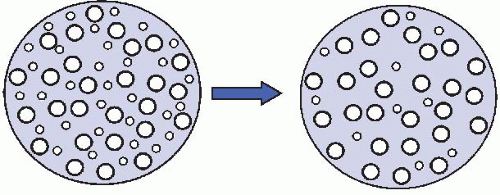
Morphometry provides quantitative data concerning axon number and axon size-frequency distribution. Large axons are lost in uremia, abetalipoproteinemia, thallium, arsenic, acute intermittent porphyria, cisplatin, vincristine, and Friedreich ataxia. Small axons are selectively damaged (Fig. 42.1) in amyloidosis, some forms of diabetic neuropathy, acute pandysautonomia, Fabry disease, and hereditary sensory and autonomic neuropathies (HSANs).
II. THREE BASIC PATHOLOGIC MECHANISMS CHARACTERIZE NEUROPATHIES
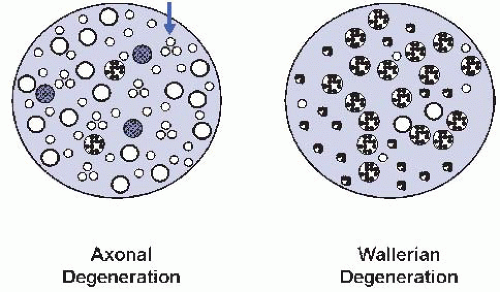
A. Axonal degeneration is the most common pattern in biopsies, resulting in degeneration of the axon and its myelin sheath (e-Figs. 42.8 and 42.9). Often the distal portions of the longest axons are preferentially involved (i.e., distal axonopathy or dying-back neuropathy). Myelin destruction and its early catabolism occurs in the Schwann cell, producing the myelin “ovoid” of teased fibers and is subsequently continued in hematogenously and endogenously derived macrophages which engulf the debris. Early regenerative events begin immediately with the proliferation of Schwann cells and their processes which accumulate within the original basal lamina of the axon/Schwann cell unit as “bands of Büngner” which are conduits for regenerating axonal sprouts. Schwann cells increase their synthesis of growth factors as trophic and tropic stimuli. Maturing axons form regenerative clusters (e-Fig. 42.10) of thinly myelinated axons within the original Schwann cell’s basal lamina and, with time, one axon emerges and begins to function and the others regress. Such a regenerated axon characteristically has a myelin sheath relatively thin for its axon’s caliber. Teased fiber preparations show a distinctive and uniform internodal length regardless of axon diameter. The end-stage of a chronic neuropathy may consist of rare preserved axons, scattered fibroblasts, and Schwann cells and may provide little information concerning the process which preceded it.
AD shares mechanisms with Wallerian degeneration (WD) which is the reaction of axons and myelin distal to a crush injury. In WD, many axons show simultaneous involvement and are often at the same histologic stage; in AD, it is typical to find degenerating, regenerating, and normal axons together, some of which may have a distinctive pathologic signature (Fig. 42.2).
B. Segmental demyelination represents preferential damage to one or several internodes of the myelin sheath, directly to myelin or to its Schwann cell, with relative axonal sparing as seen in this teased fiber preparation (e-Fig. 42.11). Schwann cell proliferation results in the replacement of each lost myelin internode by several shorter internodes resulting in variation in internodal length along individual teased fibers.
C. Secondary demyelination preferentially, nonrandomly involves selected axons, which may be atrophic or damaged, while entirely sparing others. It may reflect an abnormal axon-Schwann cell interaction resulting in secondary myelin loss. Uremic neuropathy is the prototype.
III. TOXIN-INDUCED NEUROPATHIES. A huge number of toxins and drugs (Table 42.1), including many whose neuropathic toxicity limits their clinical usefulness, produce neuropathy, likely with different mechanisms. One group of ADs targets axonal transport, and others selectively affect neurofilaments or microtubules. Acrylamide can result in distinctive neurofilament and tubulovesicular aggregates involving distal portions of axons. Zinc pyridinethionine and bromophenylacetylurea preferentially target reversal of the polarity of axonal transport resulting in terminal swellings. Lead, diphtheria toxin, perhexiline, lysolecithin, and hexachlorophene are prominent toxins preferentially directed at the Schwann cell/myelin sheath, resulting in demyelination. Many therapeutic agents may induce peripheral nerve disease. Heavy metals (e.g., arsenic, mercury, thallium, gold) are well known for their toxic effects on peripheral nerves. Neuropathic industrial and environmental agents have resulted in epidemics of toxic neuropathy.
IV. ISCHEMIC NEUROPATHIES. The nerve vascular supply (i.e., vasa nervorum) is rich and anastomotic, requiring a substantial decrease in nerve blood flow to interrupt function. Vasculitis (i.e., vasculopathy with angionecrosis) is frequently patchy, resulting in asymmetric nerve involvement (mononeuritis multiplex), emphasizing the need for thorough sampling of the nerve biopsy. Polyarteritis nodosa, the vasculitic prototype, is characterized by epineurial arteries damaged by polymorphonuclear leukocytes, macrophages, monocytes, fibrin (e-Figs. 42.12 and 42.13), and a range of axonopathy rarely culminating in infarction. There may be interor intrafascicular variability in axon loss (Fig. 42.3, and e-Figs. 42.14 and 42.15). Fibrotic recanalized vessels mark previous sites of vasculitic damage.
Patients with collagen vascular diseases may clinically present with mononeuritis multiplex, histologically comparable to that of polyarteritis nodosa or a minimal epineurial perivascular mononuclear cell infiltrate (i.e., microvasculitis) lacking angionecrosis. Epineurial perivascular collections of a few mononuclear cells are common, and some authors consider them, in isolation, to be of little pathologic importance in the absence of loss of vascular continuity or vasculopathy. Alternatively, they may represent an early or mild vascular injury, or represent
an area adjacent to more substantial vasculopathy. Immunoglobulin and complement deposition within the vascular wall in collagen vascular diseases may reflect the deposition of circulating immune complexes not specific for nerve.
TABLE 42.1 Partial List of Agents Causing Toxic Neuropathies
Metals
Toxins
Drugs
Aluminum
Acrylamide
Almitrine
Arsenic
Allyl chloride
Amiodarone
Cadmium
Buckthorn
Bortezomib
Gold
Carbon disulfide
Chloroquine
Lead
Dimethylaminopropionitrile
Cisplatin
Lithium
Dioxin
Clioquinol
Mercury
Ethanol
Colchicine
Tellurium
Ethylene oxide
Cyanide
Thallium
Hexacarbons and solvents
Dapsone
Latrotoxin
Dichloroacetate
Organophosphorus esters
Disulfiram
Perchloroethylene
Doxorubicin
Styrene
Ethambutol
Toluene
Etoposide
Toxic oil syndrome
Glutethimide
Trichloroethylene
Hydralazine
Vacor
Isoniazid
Vinyl chloride
Metronidazole
Xylene
Misonidazole
Nitrofurantoin
Nitrous Oxide
Nucleosides
Perhexiline
Phenytoin
Podophyllin
Polychlorinated biphenyls
Procainamide
Pyridoxine (Vitamin B6)
Sodium cyanate
Statins
Suramin
Tacrolimus (FK506)
Taxanes [Paclitaxel (Taxol), docetaxel]
Thalidomide
Vinca alkaloids (Vincristine, Vinblastine)
Zinc pyridinethionine

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nerve Biopsies
Nerve Biopsies
Robert E. Schmidt
Successful use of nerve biopsy requires a discussion between the clinician and neuropathologist since the clinical differential diagnosis may dictate an unusual sampling scheme, for example, vasculitis in which multiple cross-sections may be required for diagnosis.