Fig. 18.1.
Steatocystoma (A, B).
Clinical
♦
May occur in a solitary form (simplex) or as multiple lesions inherited in an autosomal dominant fashion (multiplex)
Microscopic
♦
An irregular, collapsed, intradermal cyst lined by a stratified squamous epithelium with an irregular, corrugated internal cuticle. Sebaceous glands are usually evident in the duct walls, and the cyst contains proteinaceous debris but no keratin
Dermoid Cyst
Clinical
♦
An embryonic closure defect that typically involves the skin lateral to the eye, the scalp, the neck, or near the mastoid process. Usually detected early in life
Microscopic
♦
A unilocular dermal or subcutaneous cyst lined by stratified squamous epithelium and having hair follicles, glands, and, sometimes, smooth muscle in the cyst wall
Cysts Associated with Branchial Cleft Deformities
Clinical
♦
Cystic lesions may be formed in and around the ear in association with branchial cleft deformities. These differ from the branchial cleft cyst of the neck by their location and their microscopic characteristics
Microscopic
♦
May be similar to an epidermoid cyst except for their tendency to collapse and assume a multiloculated appearance. Other forms, in addition to the above, have adnexal structures and even cartilage within their walls
Branchial Cleft Cyst
Clinical
♦
A developmental anomaly presenting as a cyst in the lateral aspect of the neck
Microscopic
♦
A lymphoepithelial cyst characterized by a stratified squamous or a pseudostratified ciliated lining with a dense lymphocytic infiltrate with germinal centers in the wall
Eruptive Vellus Hair Cyst
Clinical
♦
Small flesh-colored papules in children and young adults
Microscopic
♦
An epidermoid-like cyst that contains numerous, small vellus hairs
Pigmented Terminal Hair Cyst
Microscopic
♦
An epidermoid-like cyst containing numerous pigmented, terminal hairs
Trichilemmal (Pilar) Cyst
Clinical
♦
Dome-shaped papules/nodules found predominantly on the scalp; may be single or multiple
Microscopic
♦
A dermal or subcutaneous cyst lined by an eosinophilic stratified squamous epithelium that lacks a granular layer. The cyst contents are composed of solid, nonlaminated keratin
Proliferating Trichilemmal Cyst/Tumor (Fig. 18.2)
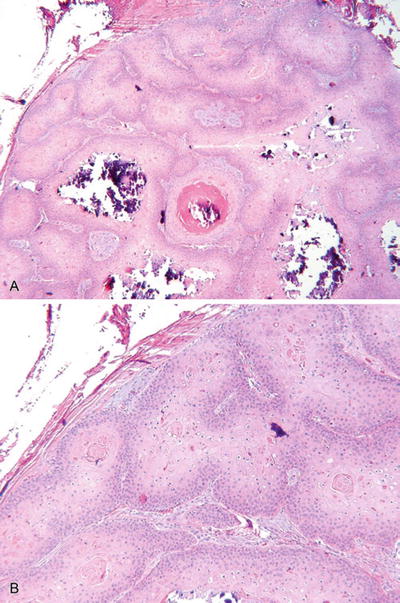
Fig. 18.2.
Proliferating trichilemmal cyst/tumor (A, B).
Clinical
♦
Multinodular scalp lesion more common in females
Microscopic
♦
Well-defined, multilobular tumor with trichilemmal keratinization; dense fibrous tissue surrounds the individual lobules; cystic areas may be inconspicuous
♦
Malignant forms occur but are rare
♦
More marked infiltration, cytologic atypia, and mitotic activity characterize the malignant variants
Bronchogenic Cyst
Clinical
♦
A developmental cyst usually found near the precordium early in life
Microscopic
♦
This cystic lesion attempts to recapitulate the bronchi with a cyst lined by respiratory epithelium and a cyst wall with smooth muscle, glands, and/or cartilage
Thyroglossal Duct Cyst
Clinical
♦
A developmental cyst found in the midline of the neck, near the hyoid bone
Microscopic
♦
The cyst may be lined by cuboidal, columnar, or stratified squamous epithelium
♦
The cyst wall contains thyroid follicles with or without skin appendages and lymphocytic inflammation
♦
Smooth muscle and cartilage are absent
Cutaneous Ciliated Cyst
Clinical
♦
Usually found on the lower extremities or buttocks of reproductive-aged women
Microscopic
♦
Ciliated, cuboidal, to columnar lined, multiloculated cyst surrounded by fibrous tissue
♦
No endometrial or fallopian tube-type stroma is evident within the cyst walls
Endometriosis and Endosalpingiosis
Clinical
♦
Blue-red cysts/nodules most commonly seen in the vulvar or periumbilical regions of reproductive-aged females
Microscopic
♦
Similar to the cutaneous ciliated cyst, except that the cyst wall contains endometrial/fallopian tube-type stroma with or without hemosiderin deposition
Hidrocystoma
Microscopic
♦
Unilocular or multilocular cysts lined by either apocrine or eccrine epithelium
Apocrine: decapitation secretion (apical snouts) and a myoepithelial layer; may have papillary projections
Eccrine: a two-layered cuboidal epithelium with no myoepithelial layer or decapitation secretion
Hybrid Cyst
Microscopic
♦
A cystic lesion combining the histologic features of more than one cyst type, usually trichilemmal and epidermoid cysts
Digital Mucous Cyst (Fig. 18.3)
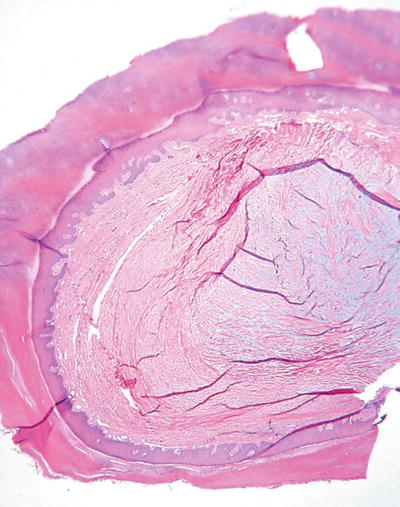
Fig. 18.3.
Digital mucous cyst.
Clinical
♦
A fluctuant, sometimes tender, translucent nodule of the digits
Microscopic
♦
Dermal mucin deposited into a cyst-like space that may or may not also contain fibroblasts and collagen
♦
There is no true epidermal lining, and the cystic spaces may be multiloculated
Oral Mucocele
Clinical
♦
A translucent, blue nodule usually found on the lower lip
Microscopic
♦
A cystic space containing varying degrees of central mucin and lined by chronic inflammatory cells with numerous foamy histiocytes
♦
There is no true epithelial lining
Epidermal Tumors and Proliferations
Actinic Keratosis (Senile, Solar)
Clinical
♦
White-yellow, erythematous, and scaly patches or plaques on sun-damaged skin; some may be pigmented
Microscopic
♦
While a variety of histologic types exist, all have in common epidermal dysplasia, which may also involve the hair follicles
♦
Hyperplastic, atrophic, acantholytic, epidermolytic, lichenoid, pigmented, bowenoid, and clear cell categories exist and reflect additional alterations to the dysplastic epidermis (e.g., lichenoid variant = actinic keratosis with a band-like lymphocytic inflammatory infiltrate; bowenoid variant = actinic keratosis with full-thickness dysplasia = carcinoma in situ)
Benign Lichenoid Keratosis (Fig. 18.4) (Lichen Planus-Like Keratosis)
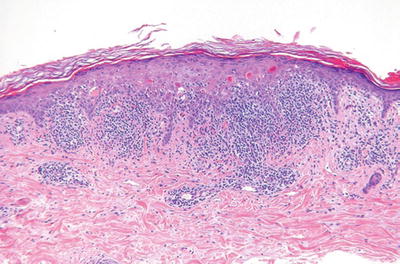
Fig. 18.4.
Benign lichenoid keratosis.
Clinical
♦
Solitary papule or plaque found primarily on the trunk or upper extremities
Microscopic
♦
Very similar to lichen planus with a dense band-like lymphocytic infiltrate at the dermal–epidermal interface with basilar vacuolar degeneration and cytoid bodies
♦
In contrast to lichen planus, eosinophils and parakeratosis may be seen
♦
In contrast to a lichenoid actinic keratosis, there is no keratinocyte dysplasia
Seborrheic Keratosis
Clinical
♦
Brown, elevated, and sharply demarcated lesions which occur most commonly on the face, trunk, and upper extremities
♦
These benign tumors often have a “stuck-on” appearance and are more common in middle-aged and older adults
♦
The sudden appearance of numerous seborrheic keratosis in association with visceral malignancy is referred to as the Leser–Trelat sign
Microscopic
♦
An epidermal proliferation of bland basaloid and polygonal keratinocytes associated with prominent keratin cyst formation
♦
The lesion is sharply delineated at its base and appears to grow up from the epidermis
♦
Irritated forms demonstrate more endophytic growth and form numerous squamous eddies
♦
Acanthotic, adenoid, clonal, pigmented, and hyperkeratotic variants exist
Inverted Follicular Keratosis (Fig. 18.5)
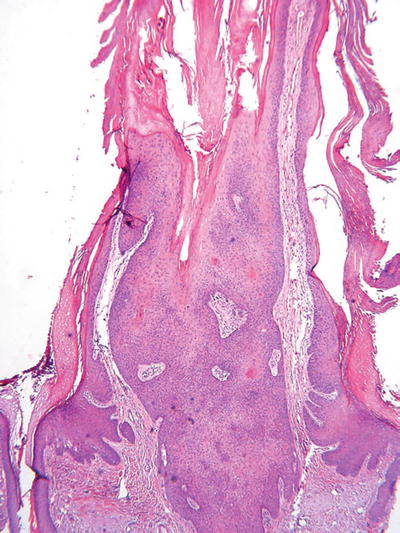
Fig. 18.5.
Inverted follicular keratosis.
Microscopic
♦
An endophytic epidermal growth with numerous squamous eddies which, like seborrheic keratosis, is sharply delineated but is centered on the hair follicles
♦
Note: This entity is considered a variant of seborrheic keratosis by some experts and a viral-induced lesion by others
Warty Dyskeratoma (Fig. 18.6)
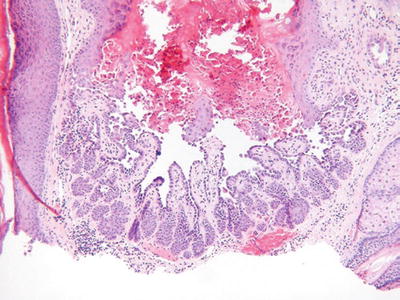
Fig. 18.6.
Warty dyskeratoma.
Clinical
♦
A benign, solitary, umbilicated nodule or papule on sun-exposed skin
Microscopic
♦
A hair follicle-centered, endophytic squamous proliferation which is sharply delineated
♦
The base of the lesion typically reveals elongated trabeculae with varying degrees of dyskeratosis that underlies broad areas of acantholytic dyskeratosis located immediately below a keratin-filled, central crater
Linear Epidermal Nevus
Clinical
♦
Localized and systemic forms exist and are characterized by a linear arrangement of closely set papillomatous papules
♦
The systemic form may be associated with other defects, including skeletal and central nervous system abnormalities
Microscopic
♦
Both variants demonstrate epidermal papillomatosis, acanthosis, and hyperkeratosis
♦
Many histologic variants exist, but, importantly, the presence of epidermolytic hyperkeratosis may be associated with systemic involvement
Nevus Comedonicus
Clinical
♦
Comedo-like papules with a central keratin plug usually in a linear arrangement on the palms, soles, or other sites
Microscopic
♦
Deep epidermal invaginations with laminated keratin similar to a comedo
♦
Epidermolytic hyperkeratosis may be evident
White Sponge Nevus
Clinical
♦
Extensive, white patches and plaques involving mucosal sites (chiefly oral but also vaginal, rectal, and esophageal) which are evident early in life and are inherited in an autosomal dominant pattern
Microscopic
♦
There is acanthosis and pallor of the mucosal lining due to prominent cytoplasmic clearing (intracellular edema) of the suprabasilar keratinocytes
♦
Similar changes are seen in leukoedema and pachyonychia congenita
Leukoedema
Clinical
♦
Patchy white plaques and patches of the oral mucosa with an adult onset
♦
These lesions remit and recur and are not inherited
Microscopic
♦
Similar to white sponge nevus from which it differs by clinical grounds
Geographic Tongue (Lingua Geographica )
Clinical
♦
Irregular, erythematous patches with white borders on the tongue
Microscopic
♦
The erythematous areas show loss of the normal granular and horny layers, while the white areas demonstrate acanthosis with neutrophilic inflammation
Clear Cell Acanthoma (Fig. 18.7)
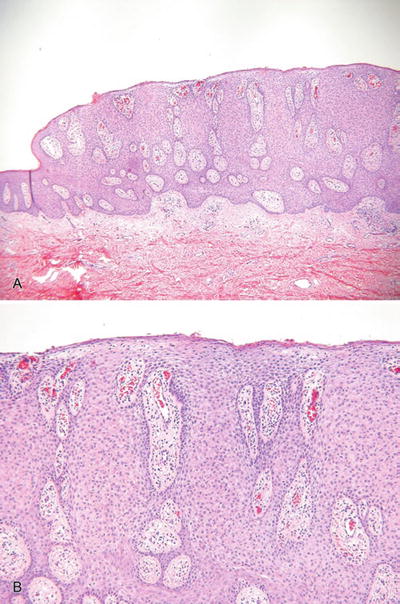
Fig. 18.7.
C lear cell acanthoma (A, B).
Clinical
♦
Solitary, slowly growing nodules or plaques with an oozing surface
♦
These lesions are most common on the lower extremities
Microscopic
♦
A platelike epidermal thickening by a proliferation of clear, heavily glycogenated keratinocytes
♦
The tumors are sharply demarcated from the adjacent epidermis (eyeliner sign) and, characteristically, have neutrophils scattered throughout the proliferation, an important feature in separating this entity from other tumors with a platelike growth pattern
Large Cell Acanthoma
Clinical
♦
Erythematous patches on sun-exposed skin
Microscopic
♦
A sharply defined epidermal proliferation of enlarged, pale keratinocytes with mild dysplasia
♦
Comment: these lesions are aneuploid and are best considered to be a variant of actinic keratosis
Squamous Cell Carcinoma In Situ (Bowen Disease )
Clinical
♦
Erythematous, irregular, scaly patches, and plaques that may involve any skin surface as well as the mucous membranes
♦
Bowen disease of the penis is often referred to as erythroplasia of Queyrat
♦
Sun exposure, arsenic, and other chemicals are associated with an increased risk of development of Bowen disease
♦
Approximately 5% of these lesions develop an invasive component
Microscopic
♦
Epidermal acanthosis associated with full-thickness epidermal dysplasia, which may involve the adnexa
♦
A dense lichenoid inflammatory infiltrate may also be present
♦
Intraepidermal spread (Borst–Jadassohn phenomena) may be prominent and should be differentiated from melanoma and Paget disease
Bowenoid Papulosis
Clinical
♦
Red-brown papules or plaques on the external genitalia and perineum of young adults
♦
Lesions are frequently multiple, and there is a strong association with human papillomavirus (HPV) types 16 and 18 with other types occurring less frequently
♦
Unlike Bowen disease, these lesions may regress and are less likely to give rise to an invasive carcinoma
Microscopic
♦
Fairly discrete areas of epidermal acanthosis associated with varying degrees of epidermal dysplasia, koilocytosis, hypergranulosis, and parakeratosis
♦
In situ carcinoma may be present
Squamous Cell Carcinoma (Fig. 18.8)
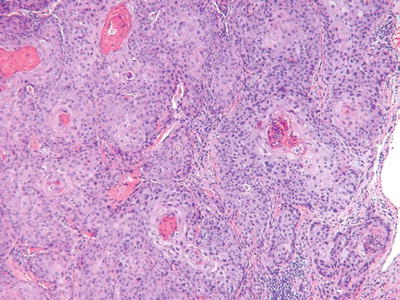
Fig. 18.8.
S quamous cell carcinoma.
Clinical
♦
Indurated, hyperkeratotic nodules which may show ulceration or verruciform changes
♦
Currently, squamous cell carcinoma is the second most common cutaneous malignancy
♦
The incidence is increasing
♦
While most are related to sun exposure, other risk factors include fair complexion, chronic inflammation, immunosuppression, burns, HPV infection, and chemical exposure (e.g., arsenic)
♦
The overall rate of metastases is approximately 5% but is >10% at mucosal sites and the ear
Microscopic
♦
Atypical nests of epidermoid cells invasive into the dermis and, usually, with overlying epidermal dysplasia
♦
Differentiation varies from poorly differentiated (minimal keratin production, marked nuclear pleomorphism, high mitotic rate) to well differentiated (abundant keratin pearl formation, minimal cytologic atypia, and few mitoses)
♦
The presence of keratin production, intercellular desmosomes (“spines”), and overlying epidermal dysplasia are useful features in separating this tumor from other entities
♦
Variants include clear cell, spindle cell, acantholytic, and verrucous tumors
Verrucous Carcinoma
Clinical
♦
A variant of squamous cell carcinoma that typically appears as a large hyperkeratotic nodule
♦
All cutaneous surfaces may be involved but plantar, oral (oral florid papillomatosis), and anogenital lesions are particularly common
♦
These tumors frequently recur but have little metastatic potential
Microscopic
♦
An endophytic and exophytic growth of well-differentiated squamous epithelium with extensive keratinization
♦
The deep component shows a broad, pushing front at its advancing edge
♦
If any significant nuclear dysplasia is present, a diagnosis of squamous cell carcinoma should be made
Keratoacanthoma
Clinical
♦
A rapidly growing, umbilicated nodule with a central keratin plug
♦
Multiple lesions may be present
♦
Typically, these tumors regress over the course of a few months, but they may recur
♦
These tumors are considered to be a variant of squamous cell carcinoma by some experts
Microscopic
♦
A well-defined, sharply demarcated, crateriform squamous proliferation with a central keratin plug
♦
The squamous epithelium frequently has a glassy appearance and lacks significant cytologic atypia
♦
A lichenoid inflammatory infiltrate may be present
♦
The presence of cytologic atypia, infiltrative borders, or atypical mitoses warrants a diagnosis of a squamous cell carcinoma
Pilar and Pilosebaceous-Derived Tumors
Dilated Pore of Winer
Clinical
♦
Flesh-colored papule or cyst with a central keratotic plug found chiefly on the head and neck
Microscopic
♦
A cone-shaped dilatation of the follicular infundibulum with a central keratin plug
♦
The wall of the pore is proliferative with fingerlike projections extending into the adjacent dermis
♦
No secondary hair follicles are evident within the cyst wall
Pilar Sheath Acanthoma
Clinical
♦
Small nodule with a central keratin-filled pore on the upper lip
Microscopic
♦
Similar in architecture to the dilated pore but with a more proliferative wall
♦
The epithelium of the wall is paler than that of the dilated pore and may have some degree of peripheral palisading, suggesting abortive hair follicle development
♦
No well-developed secondary hair follicles with hair formation are evident
Trichofolliculoma (Fig. 18.9)
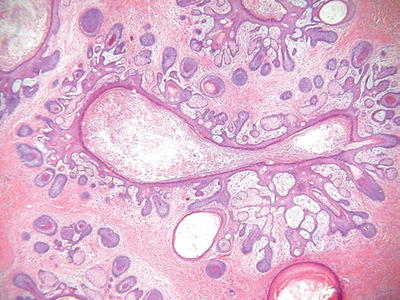
Fig. 18.9.
Trichofolliculoma.
Clinical
♦
Solitary, flesh-colored nodules with a central pore from which numerous white hairs emerge
Microscopic
♦
Similar to the dilated pore, a central, elongated, and dilated infundibulum is present
♦
Numerous secondary hair follicles radiate peripherally from the central cavity that is filled with laminated keratin and numerous hairs
♦
If sebaceous glands are evident within the secondary follicles, then a diagnosis of a sebaceous trichofolliculoma is appropriate
Tumor of the Follicular Infundibulum (Fig. 18.10)
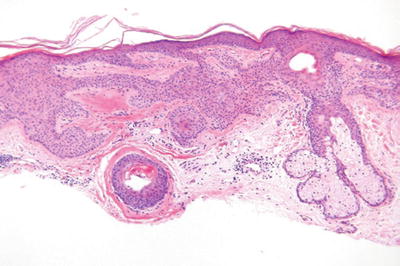
Fig. 18.10.
Tumor of the follicular infundibulum.
Clinical
♦
Small, hyperkeratotic papules or plaques on the head and neck
Microscopic
♦
A platelike expansion of the epidermis by interanastomosing and interweaving trabeculae of glycogenated squamous epithelium within the superficial dermis
♦
The trabeculae show multiple attachments to the epidermis and to the hair follicles
♦
The trabeculae may show some peripheral palisading but lack mucin deposition or stromal retraction
♦
Elastic fibers are often condensed at the base of the lesion
Differential Diagnosis
♦
Fibroepithelioma of Pinkus and eccrine syringofibroadenoma
♦
Fibroepithelioma of Pinkus has narrow trabeculae with more pronounced basaloid differentiation, while eccrine syringofibroadenoma shows scattered eccrine ducts within its trabeculae
Basaloid Follicular Hamartoma
Clinical
♦
Small, flesh-colored papules or plaques on the head and neck
♦
Solitary, multifocal, and inherited variants have been described
Microscopic
♦
Small, starfish- or octopus-like proliferations of basaloid cells within the dermis arranged as anastamosing trabeculae with peripheral palisading and surrounding fibrosis
Trichilemmoma (Fig. 18.11)
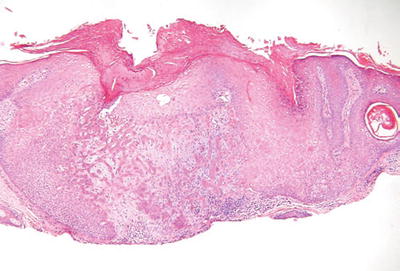
Fig. 18.11.
T richilemmoma.
Clinical
♦
Verrucous, hyperkeratotic papules usually found on the face
♦
Multiple trichilemmomas occur in the autosomal dominant disorder, Cowden disease
Microscopic
♦
A single lobule or, occasionally, a multilobular proliferation of round, clear (glycogen-rich) squamous cells giving a platelike thickening to the epidermis
♦
There is usually a follicular accentuation to the proliferation with the pale cells growing down preexisting follicular structures
♦
The lobules are surrounded by a PASD-positive basement membrane
♦
A desmoplastic variant exists which, in addition to typical trichilemmoma areas, has central trabeculae surrounded by a dense, hypocellular stroma
Trichoadenoma (Fig. 18.12)
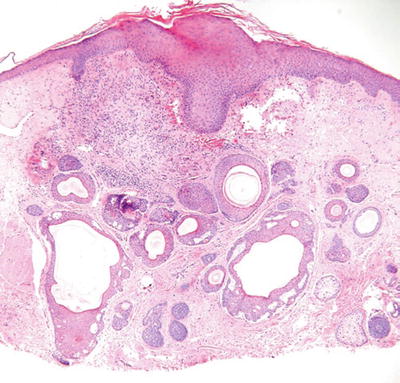
Fig. 18.12.
Trichoadenoma.
Clinical
♦
Yellow to flesh-colored papule on the face
Microscopic
♦
Numerous keratin-filled cysts lined by a stratified squamous epithelium are evident within the dermis
♦
A granular layer is present (epidermoid keratinization), and the cysts have a surrounding fibrous stroma
♦
Solid trabeculae are rare
Trichoepithelioma
Clinical
♦
Small flesh-colored papules on the face of young- to middle-aged adults
♦
Solitary, desmoplastic, and multiple (autosomal dominant inheritance) variants exist
Microscopic
♦
An admixture of keratin-filled cysts and trabeculae of basaloid cells with peripheral palisading and a surrounding fibrous stroma
♦
Stromal retraction is not evident, and true hair bulb formation is rarely seen
Differential Diagnosis
♦
Keratotic basal cell carcinoma is different from trichoepithelioma by having stromal retraction, mucin deposition, individual cell necrosis, and numerous mitoses
♦
Microcystic adnexal carcinoma differs from desmoplastic trichoepithelioma by the presence of deeper infiltration with eccrine ducts lined by an eosinophilic cuticle. A layered appearance with cysts predominating superficially and trabeculae predominating at the deep aspect of the tumor are characteristic
Trichoblastoma
Clinical
♦
A controversial entity having histologic and clinical overlap with trichoepithelioma and basal cell carcinoma
Microscopic
♦
A proliferation of germinative basaloid cells arranged in nests, sheets, or trabeculae
♦
Conspicuous hair bulb differentiation is seen at the edge of the nests or sheets but also as single, primitive hair follicle-like structures surrounded by a dense fibrous sheath
♦
Stromal clefting and extensive mucin deposition are typically absent
Differential Diagnosis
♦
Basal cell carcinoma shows stromal clefting, mucin deposition, and single cell necrosis and lacks primitive hair bulb structures
♦
Trichoepithelioma has admixed keratin-filled cysts and has few to no primitive hair bulbs
Trichodiscoma
Clinical
♦
A hamartomatous proliferation of the hair disk which presents as multiple flesh-colored papules on the face and also elsewhere on the body
Microscopic
♦
A nodular mesenchymal proliferation surrounded by an epidermal collarette
♦
Centrally there are stellate fibroblasts embedded in collagen, reticulin, and elastic fibers with abundant mucin deposition
♦
Thin-walled vessels with prominent basement membranes are seen within the proliferation
Perifollicular Fibroma and Fibrofolliculoma
Clinical
♦
Both occur as solitary and, more often, multiple flesh-colored papules on the face or neck
Microscopic
♦
Perifollicular fibromas show a loose, concentric proliferation of fibrous tissue around normal hair follicles, while fibrofolliculomas show both a fibrous and a follicular proliferation centered on a dilated follicle
♦
The epithelial component of the latter consists of epithelial trabeculae that arise from the infundibulum and are surrounded by fibrous tissue
Pilomatricoma (Fig. 18.13) (Calcifying Epithelioma of Malherbe)
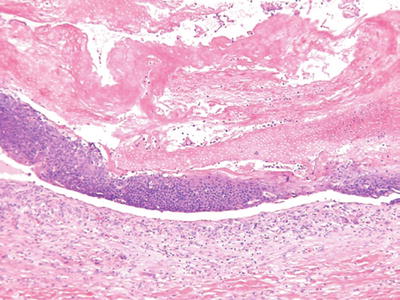
Fig. 18.13.
Pilomatricoma.
Clinical
♦
Deep-seated, frequently calcified nodules on the head, neck, and upper extremities of children and young adults
♦
These lesions may be solitary or multiple (autosomal dominant inheritance) or may be a marker of a systemic disease (Gardner syndrome)
Microscopic
♦
A cystic or multinodular tumor with a biphasic epithelial growth pattern consisting of eosinophilic, ghosts, or shadow cells centrally and basophilic, basaloid cells peripherally
♦
Granulomatous inflammation and calcification are frequent and may obscure the characteristic growth pattern
Basal Cell Carcinoma (Fig. 18.14)
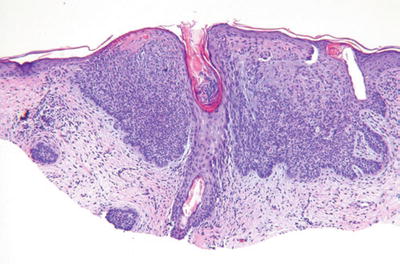
Fig. 18.14.
Ba sal cell carcinoma.
Clinical
♦
Well-delineated, pearly, translucent, pink-tan papules or nodules with telangiectasia
♦
Superficial, nodular/ulcerative, pigmented, diffuse, morpheaform, and fibroepitheliomatous variants exist
♦
Most are found on sun-exposed skin of the elderly, but occasional cases are evident on non-sun-exposed skin
♦
Basal cell carcinoma is currently the most common cutaneous malignancy, and the incidence is increasing
♦
Risk of developing basal cell carcinomas is related to sun exposure and skin type
♦
Multiple tumors are seen in Bazex syndrome and basal cell carcinoma nevus syndrome (Gorlin syndrome), an autosomal dominant inherited disease also having odontogenic keratocysts, palmar-plantar pits, ectopic calcification, and skeletal abnormalities
♦
Basal cell carcinoma has little tendency to metastasize
Microscopic
♦
A proliferation of atypical basaloid cells in nests, trabeculae, and/or cysts within the dermis but often demonstrating multifocal epidermal attachment
♦
Peripheral palisading, stromal retraction, mucin deposition, single cell necrosis, and mitoses are characteristic and are useful in separating this tumor from other entities
♦
Nodulocystic, metatypical (keratotic), pigmented, adenoidal, infiltrating, superficial, and morpheaform histologic variants exist, with the latter two having an increased risk of recurrence
Fibroepithelioma of Pinkus (Fig. 18.15)
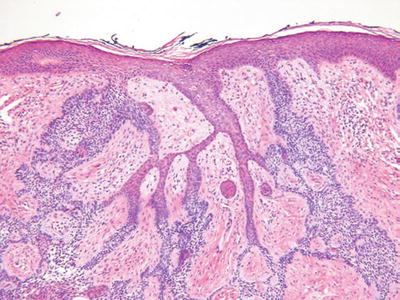
Fig. 18.15.
Fibroepithelioma of Pinkus.
Clinical
♦
Polypoid or plaque-like lesions on the thigh or trunk
♦
Considered as a premalignant lesion by many experts
Microscopic
♦
Interanastomosing epithelial strands with multiple points of attachment to the epidermis and surrounded by a fibrous stroma
♦
The trabeculae are thinner than the tumor of the follicular infundibulum, being two to three epithelial cells in thickness
Malignant Pilomatricoma
Clinical
♦
A rare tumor occurring as tumors or nodules on the face
Microscopic
♦
Similar to a benign pilomatricoma but showing areas with infiltration, mitoses, and nuclear pleomorphism
Nevus Sebaceous (Fig. 18.16)
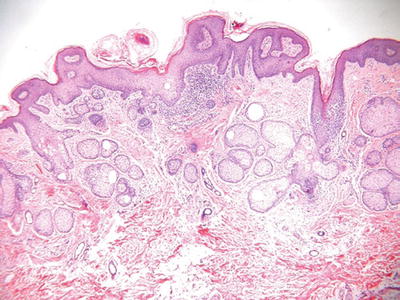
Fig. 18.16.
Nevus sebaceous.
Clinical
♦
Single or multiple, yellow papules to plaques with or without verruciform features on the head and neck of infants, adolescents, and young adults
♦
A linear form exists
♦
Nevus sebaceous with cerebral abnormalities is referred to as the nevus sebaceous syndrome
♦
Basal cell carcinoma is the most common malignancy associated with nevus sebaceous, while syringocystadenoma papilliferum is the most common benign proliferation associated with this condition
Microscopic
♦
The epidermis frequently demonstrates papillomatosis or verruciform change
♦
Numerous immature or abortive hair follicles are situated within the superficial dermis with a reduction in the number of mature terminal hairs
♦
The sebaceous glands appear haphazardly distributed within the dermis and may appear atrophic, hyperplastic, or relatively normal in size
♦
Apocrine glands are a frequent finding in the deep dermis
Sebaceous Hyperplasia
Clinical
♦
Small yellow papules on the face and forehead of older adults
Microscopic
♦
Enlarged, hyperplastic sebaceous glands emptying into a central hair follicle often situated in the superficial dermis
Sebaceous Adenoma
Clinical
♦
Pink to yellow papules on the face and neck of older adults
♦
Multiple lesions are often associated with visceral malignancy (Muir–Torre syndrome)
Microscopic
♦
A multilobulated tumor often showing attachment to or emptying through the overlying epidermis
♦
The lobules are composed of basaloid cells peripherally and multivacuolated cells centrally
♦
By definition, the basaloid cells comprise less than 50% of cells of the individual lobules
♦
Infiltration, necrosis, and frequent mitoses are absent
Sebaceous Epithelioma
Clinical
♦
Similar to sebaceous adenoma
Microscopic
♦
A faintly lobular tumor similar to the sebaceous adenoma
♦
Basaloid cells comprise more than 50% of the cells in the individual lobules
♦
While occasional mitotic figures may be seen, abundant mitotic activity, infiltration, nuclear pleomorphism, or necrosis should lead to a consideration of sebaceous carcinoma or basal cell carcinoma with sebaceous differentiation
Sebaceous Carcinoma (Fig. 18.17)
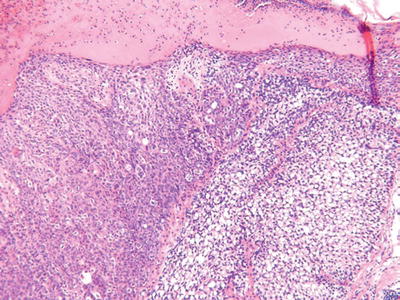
Fig. 18.17.
Sebaceous carcinoma.
Clinical
♦
Ulcerated or nonulcerated nodules on the head and neck region of older adults
♦
The periocular region is a particularly common site where derivation from the meibomian gland occurs
♦
These tumors are associated with a high metastatic potential and increased mortality (~25%)
Microscopic
♦
These tumors may show irregular lobules or a diffuse infiltrating pattern
♦
Attachment to the overlying epidermis may be present, and pagetoid spread is common in the periocular variants
♦
These tumors show a spectrum of sebaceous differentiation varying from tumors composed predominantly of basaloid cells to tumors with numerous multivacuolated sebaceous cells
♦
Infiltration, necrosis, nuclear pleomorphism, nucleoli, and mitoses are usually readily evident
♦
Perineural and capillary–lymphatic space invasion may be seen
Immunophenotype
♦
Cytokeratin and EMA+; S-100 protein and CEA–
Eccrine-Derived Tumors and Proliferations
Syringoma-Like Proliferations Associated with Alopecia
Clinical
♦
No specific clinical findings are associated with this lesion which presents simply as alopecia of any etiology
Microscopic
♦
This is a relatively rare, apparently nonneoplastic proliferation of the eccrine ducts in response to alopecia
♦
The microscopic characteristic is that of the particular form of alopecia affecting the patient with the addition of a diffuse, haphazard proliferation of the eccrine ducts limited to the mid and upper dermis
♦
Ducts, trabeculae, comma, and tadpole-shaped forms may all be seen
♦
No nuclear pleomorphism or perineural invasion is evident, but mitotic figures may be seen
♦
This proliferation may be seen throughout the scalp in cases of severe alopecia
Differential Diagnosis
♦
Syringoma is usually a more localized and circumscribed eccrine proliferation and lacks the haphazard appearance of the above
♦
Microcystic adnexal carcinoma and eccrine syringoid carcinoma are more infiltrative lesions that typically involve the lower dermis and the subcutis and show frequent perineural invasion
Eccrine Syringofibroadenoma (Fig. 18.18)
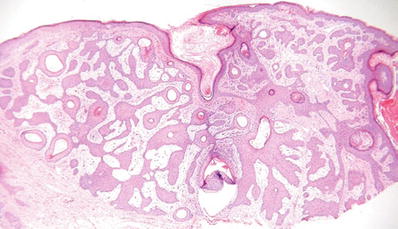
Fig. 18.18.
Eccrine syringofibroadenoma.
Clinical
♦
Single or multiple papules or nodules with a wide age range and distribution
♦
The extremities are most commonly involved
Microscopic
♦
Interanastomosing cords and trabeculae of epithelial cells extending into the dermis with multiple points of attachment to the epidermis
♦
The cords are thin and surrounded by a fibrous stroma
♦
Scattered throughout the epithelial cords are areas of eccrine duct differentiation with prominent eosinophilic cuticles
Differential Diagnosis
♦
Tumor of the follicular infundibulum and fibroepithelioma of Pinkus lack eccrine differentiation
Syringoma (Fig. 18.19)
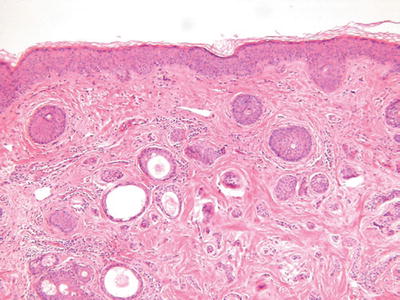
Fig. 18.19.
Syringoma.
Clinical
♦
Multiple flesh-colored or faintly yellow papules on the eyelids or upper face
♦
Other sites may also be involved, and linear and eruptive variants occur
♦
Clear cell variants may be associated with diabetes
Microscopic
♦
A fairly well-circumscribed but unencapsulated neoplasm involving the mid to upper dermis composed of eccrine-derived cords and ducts
♦
The cords may show characteristic tadpole-shaped forms
♦
Two or more cell layers with an internal eosinophilic cuticle and varying degrees of clear cell change line the ducts
♦
In general, the tumor does not infiltrate the deep dermis or subcutaneous fat, lacks mitotic activity and necrosis, and has no significant pleomorphism
Immunophenotype
♦
Eccrine-derived tumors typically stain positively for the cytokeratins, EMA, and CEA, which highlights the luminal aspect of the eccrine ducts
Differential Diagnosis
♦
Desmoplastic trichoepithelioma has numerous keratotic cysts and frequent calcification and lacks eccrine duct formation
♦
Microcystic adnexal carcinoma is much more infiltrative and typically involves the deep dermis and subcutaneous fat
Chondroid Syringoma (Benign Mixed Tumor, Fig. 18.20)
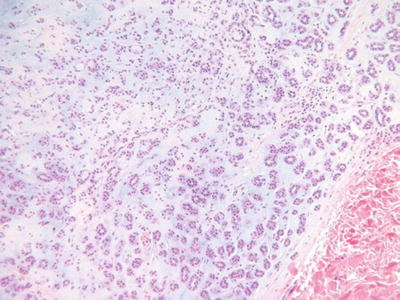
Fig. 18.20.
Chondroid syringoma.
Clinical
♦
A benign, slowly growing, typically solitary tumor nodule on the head and neck; other sites may be involved
Microscopic
♦
A well-circumscribed, biphasic tumor nodule located within the dermis and/or the subcutaneous fat
♦
Epithelial cords, trabeculae, and ducts are embedded in an abundant myxoid, fibromyxoid, or cartilaginous matrix
♦
The epithelial component lacks nuclear pleomorphism, infiltration, necrosis, and mitotic activity
♦
The ducts may show eccrine and/or apocrine differentiation
Differential Diagnosis
♦
Pleomorphic adenoma (benign mixed tumor of the salivary glands) should be differentiated from chondroid syringoma because of its tendency to recur and, possibly, malignant transformation
♦
As these tumors are similar histologically, location and the presence of adjacent normal salivary glands are the most reliable features used to separate these entities
Malignant Chondroid Syringoma
Clinical
♦
A rare, highly malignant tumor with a predilection for the distal extremities
♦
These tumors frequently recur and metastasize and are associated with increased mortality
Microscopic
♦
Malignant appearing, infiltrating epithelial cords, ducts, and sheets that overgrow the benign mesenchymal matrix
♦
Necrosis, mitoses, and nuclear pleomorphism are present
♦
An adjacent benign mixed tumor is not typically seen
Differential Diagnosis
♦
Carcinosarcoma shows a malignant mesenchymal component in addition to an epithelial malignancy
Papillary Eccrine Adenoma
Clinical
♦
A firm, pink to tan nodule on the distal extremities of adolescents and young adults with a female predominance
♦
Blacks are more commonly affected than Whites
Microscopic
♦
A fairly well-circumscribed but unencapsulated proliferation of eccrine ducts and duct-like structures within the dermis
♦
The ducts are lined by a multilayered cuboidal epithelium without apical snouts
♦
Micropapillary projections and transluminal bridging may be seen
♦
The tumor stroma is fibrotic and frequently hyalinized. Cribriform structures, necrosis, mitotic activity, and nuclear pleomorphism are absent
Differential Diagnosis
♦
Aggressive digital papillary adenoma/adenocarcinoma (see below)
Aggressive Digital Papillary Adenoma/Adenocarcinoma
Clinical
♦
Asymptomatic flesh-colored nodule on the digits of middle-aged adults
♦
The recurrence rate is approximately 50% for these tumors, and the overtly malignant lesions have a metastatic rate of approximately 25–40%
Microscopic
♦
Generally, an unencapsulated and poorly circumscribed proliferation of eccrine ducts, tubules, cysts, and nests within the dermis and/or subcutaneous fat
♦
The ducts and cystic structures are lined by a multilayered epithelium with abundant micro- and macropapillae
♦
Cribriform structures are frequently identified
♦
Varying degrees of nuclear pleomorphism, mitotic activity, and necrosis may be seen
♦
Tumor grade correlates with metastatic potential, but all forms may metastasize
♦
Note: all of these lesions are best classified as adenocarcinomas due to their propensity for recurrence
♦
A histologic grade should be given as a prognostic indicator for the risk of metastases
Differential Diagnosis
♦
Papillary eccrine adenoma does not show the infiltration, nuclear atypia, mitotic activity, or the cribriforming of the aggressive digital papillary adenocarcinoma
♦
Nodular hidradenoma (clear cell )
Hidradenoma, Solid-Cystic Hidradenoma, and Eccrine Acrospiroma (Fig. 18.21)
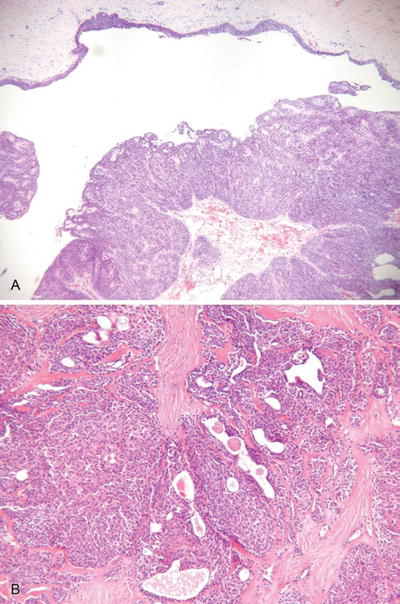
Fig. 18.21.
N odular hidradenoma (A, B).
Clinical
♦
Solid or cystic, intradermal nodule 05–2.0 cm in diameter
♦
These tumors are usually solitary but may be multiple
♦
The head, neck, and extremities are most commonly involved
♦
Predominates in young adults with a slight female predominance
Microscopic
♦
A well-circumscribed and often pseudoencapsulated tumor composed of a single lobule or, more often, multiple lobules of eosinophilic to clear cells in the dermis
♦
Cystic change may be prominent
♦
Tubular structures lined by cuboidal to columnar cells with an eosinophilic cuticle are evident in most tumors and are important in proper classification
♦
Foci of squamous and/or mucinous differentiation may be seen
♦
While this tumor is predominantly intradermal, occasional attachments may be seen to the overlying epidermis
♦
Necrosis, mitotic activity, and nuclear pleomorphism are absent or minimal in extent
Hidradenocarcinoma (Malignant Nodular Hidradenoma )
Clinical
♦
Similar distribution to their benign counterparts but occurs in older individuals (>50 years of age)
♦
Recurrence and metastatic rate is approximately 50%
Microscopic
♦
Like the hidradenoma, this tumor is composed of eosinophilic and/or clear cells forming lobules within the dermis
♦
The malignant variants, however, are asymmetrical, infiltrating, and mitotically active and demonstrate nuclear pleomorphism and/or necrosis
Differential Diagnosis
♦
Clear cell squamous cell carcinoma, clear cell renal cell carcinoma, clear cell melanoma, and trichilemmal carcinoma
♦
Look for tubular structures with an eosinophilic cuticle to confirm eccrine differentiation; CEA may be useful in that it highlights the luminal border of eccrine-derived structures
Hidroacanthoma Simplex (Intraepidermal Poroma )
Clinical
♦
A rare, benign variant of poroma typically involving the extremities of older individuals
Microscopic
♦
An intraepidermal proliferation (Borst–Jadassohn phenomenon) of round to faintly spindled cells with eosinophilic to faintly clear cytoplasm
♦
Intracytoplasmic glycogen is evident on PAS staining
♦
Rare eccrine ducts/tubules may be seen
♦
The individual cells are remarkably uniform and lack nuclear atypia
♦
The tumor cells are sharply demarcated from the squamous cells of the adjacent epidermis
Eccrine Poroma (Fig. 18.22)
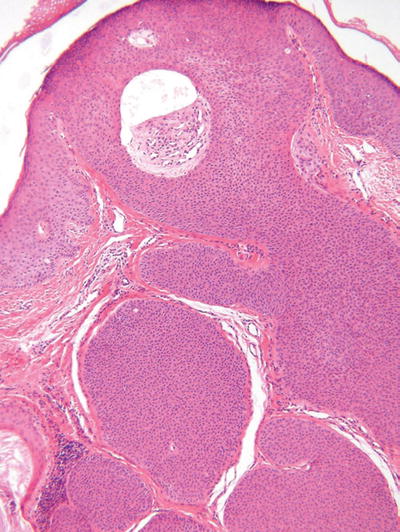
Fig. 18.22.
Eccrine poroma.
Clinical
♦
A red to flesh-colored tumor found most frequently on the sole of the foot or hand, but other sites may be involved
♦
These tumors may reach many centimeters in diameter and may be pedunculated
♦
While the majority of these tumors are solitary, multiple lesions may be seen (eccrine poromatosis)
Microscopic
♦
This tumor is composed of numerous cords or trabeculae of small rounded tumor cells which rain down from the epidermis into the dermis in a fairly circumscribed manner
♦
The epidermal component is similar to hidroacanthoma simplex, while the dermal component often shows numerous well-formed lobules with frequently conspicuous duct formation
♦
Cystic change is typically less than that seen in hidradenoma, while the degree of epidermal involvement is significantly greater
♦
The tumor cells show intercellular bridges and should not be confused with squamous cells that are larger and more polygonal
♦
Poromatous lesions that are entirely limited to the dermis are often called dermal duct tumors
Porocarcinoma (Malignant Eccrine Poroma )
Clinical
♦
The malignant counterpart to the eccrine poroma affects similar sites but tends to occur in older individuals with a long history of progressive tumor growth
♦
The recurrence and metastatic rate approaches 25%
♦
Multiple cutaneous metastases are not uncommon
Microscopic
♦
Like their benign counterparts, epidermal, dermal, and mixed epidermal–dermal variants are seen
♦
The epidermal variants and the cutaneous metastases typically show frank pagetoidosis with scattered foci of ductal differentiation
♦
The mixed variants are by far the most common and have a similar architecture to their benign counterpart; however, these tumors are infiltrative and mitotically active and demonstrate nuclear pleomorphism and occasionally perineural and capillary–lymphatic invasion
Cylindroma and Malignant Cylindroma (Fig. 18.23)
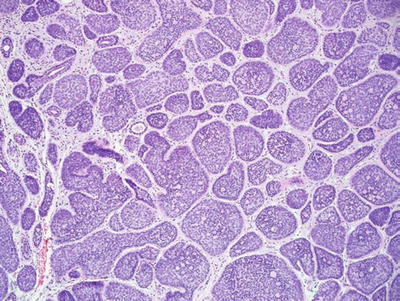
Fig. 18.23.
Cylindroma.
Clinical
♦
Solitary or multiple, red to purple nodules on the head, neck, or scalp
♦
Multiple tumors (turban tumors) are inherited in an autosomal dominant fashion and may be associated with multiple trichoepitheliomas
Microscopic
♦
Multiple, dermal-based lobules are found in the dermis and have an interlocking or “jigsaw puzzle” appearance
♦
The individual lobules are surrounded by an eosinophilic basement membrane
♦
Two cell types are evident within the lobules: a lymphocyte-like population of cells with hyperchromatic nuclei which predominate at the periphery of the lobules and a population of larger cells with oval, vesicular nuclei which predominate centrally
♦
Hylanizing basement membrane-like material is also frequently evident within the lobules
♦
Malignant variants demonstrate nuclear pleomorphism, mitotic activity, loss of the surrounding basement membrane, and infiltration of adjacent tissue
♦
Malignant variants are rare and may arise in the background of multiple benign cylindromas
Eccrine Spiradenoma and Malignant Eccrine Spiradenoma (Fig. 18.24)
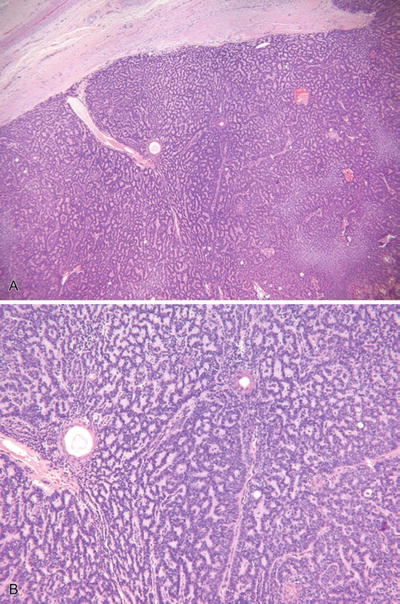
Fig. 18.24.
Eccrine spiradenoma (A, B).
Clinical
♦
Usually solitary, blue- to flesh-colored intradermal nodules on the ventral aspect of the body
♦
These tumors are frequently painful Rarely, multiple tumors may be seen in a linear or zosteriform distribution
Microscopic
♦
One or more basophilic tumor lobules are evident in the dermis and are usually encapsulated
♦
The lobules are composed of two cell types similar to the cylindroma: a small, lymphocyte-like population and a larger cell type with oval, vesicular nuclei
♦
Ductal differentiation may be conspicuous within the lobules, and pseudovascular spaces may give a hemangiomatous quality to the lesion
♦
The intervening stroma often demonstrates lymphangiectasia
♦
The rare malignant variants require an adjacent benign focus of typical microscopy for confident diagnosis
♦
The malignant variants are characterized by infiltration, mitoses, nuclear pleomorphism, necrosis, and lymphatic invasion
Eccrine Duct Carcinoma
Clinical
♦
A nodular and often ulcerated lesion of long-standing duration found most commonly on the head, neck, and extremities of older adults
♦
Approximately 50% metastasize to lymph nodes or visceral sites
Microscopic
♦
An infiltrating dermal tumor composed of strands, trabeculae, and tubules with varying degrees of lumen formation
♦
The histologic pattern is very similar to ductal carcinoma of the breast, which should always be excluded clinically
♦
There is at least some degree of nuclear pleomorphism, and nucleoli are frequently prominent
♦
Mitotic figures and necrosis may also be identified
Syringoid Eccrine Carcinoma (Eccrine Epithelioma )
Clinical
♦
Typically, a plaque or an ulcerated tumor of the scalp of middle-aged adults
♦
This tumor is locally aggressive with frequent recurrences, but metastases are rare
Microscopic
♦
A dermal-centered tumor showing extensive infiltration with the involvement of the subcutaneous fat
♦
This tumor is composed of infiltrating cords and trabeculae with faint lumen formation and a dense, hyalinizing stroma
♦
Unlike microcystic adnexal carcinoma, keratocysts are rarely present
♦
The individual cells often have basaloid features but lack peripheral retraction
♦
Nuclear pleomorphism is mild, and mitotic figures are scarce
♦
Perineural invasion is frequent
Microcystic Adnexal Carcinoma (Fig. 18.25)
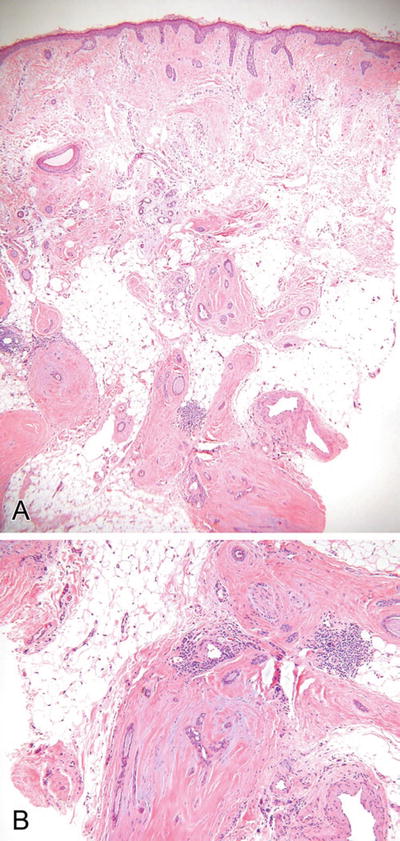
Fig. 18.25.
Microcystic adnexal carcinoma (A, B).
Clinical
♦
A flesh-colored to yellow, slowly growing firm plaque or nodule involving the head, neck, or face of older adults
♦
Like syringoid eccrine carcinoma, this is a locally aggressive tumor with frequent recurrences but with no tendency to metastasize
Microscopic
♦
A dermal-centered tumor showing extensive infiltration of the deep dermis and subcutaneous tissues
♦
Keratocysts, trabeculae, and ductules are evident throughout the lesion, but keratocysts tend to predominate superficially while trabeculae predominate at the deeper aspect of the tumor
♦
The middle of the tumor shows an admixture of all forms, giving this tumor a triphasic or trilayered look from superficial to deep
♦
The individual epithelial units are frequently invested by a dense fibrous stroma, giving this tumor a sclerotic appearance
♦
Perineural invasion is common, while nuclear pleomorphism and mitotic activity are rare
Mucinous Eccrine Carcinoma
Clinical
♦
A flesh-colored to blue nodule on the head and neck region (particularly the eyelid) of older adults with a male predominance
Microscopic
♦
A dermal-based tumor showing islands of relatively bland epithelial cells floating in pools of mucin similar to colloid carcinoma of the breast
Adenoid Cystic Carcinoma
General
♦
A rare, primary cutaneous neoplasm showing a similar microscopic characteristic to its counterparts elsewhere but having a less aggressive course
Mucoepidermoid Carcinoma
General
♦
A rare primary tumor of the skin showing similar histologic features to its salivary gland counterpart
Apocrine-Derived Tumors and Proliferations
Apocrine Nevus
Clinical
♦
A rare lesion typically presenting as a papule in the axilla or on the scalp
Microscopic
♦
An increase in the number or size of mature-appearing apocrine glands
Syringocystadenoma Papilliferum (Fig. 18.26)
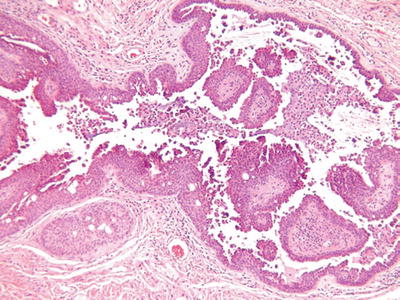
Fig. 18.26.
S yringocystadenoma papilliferum.
Clinical
♦
Typically a solitary, verrucous to papillary lesion on the scalp, face, or neck, but other sites may be involved
♦
In children, this tumor frequently arises within a nevus sebaceous
Microscopic
♦
A partially cystic, dermal-centered tumor showing overlying epidermal invagination
♦
The cystic space contains abundant papillary structures lined by a bilayered epithelium with apical snouts, consistent with apocrine differentiation
♦
The fibrovascular cores contain abundant plasma cells
Hidradenoma Papilliferum
Clinical
♦
Typically a solitary, asymptomatic nodule present in the genital region of females
♦
Similar lesions have been described within the ear, nipple, and eyelid
Microscopic
♦
A well-demarcated, dermal-based neoplasm showing no involvement of the overlying epidermis
♦
This tumor is also frequently cystic in areas and is characterized by numerous trabeculae, epithelial fronds, and papillary structures lined by a bilayered epithelium showing apocrine differentiation (apical snouts)
♦
The stroma is fibrovascular and lacks the plasma cells of syringocystadenoma papilliferum
♦
Occasional cases show a more pronounced fibrous stroma with a lobular architecture akin to fibroadenoma of the breast
♦
Rare cases show malignant transformation with high-grade nuclear features, frequent mitoses, necrosis, and infiltration
Tubular Apocrine Adenoma (Fig. 18.27)
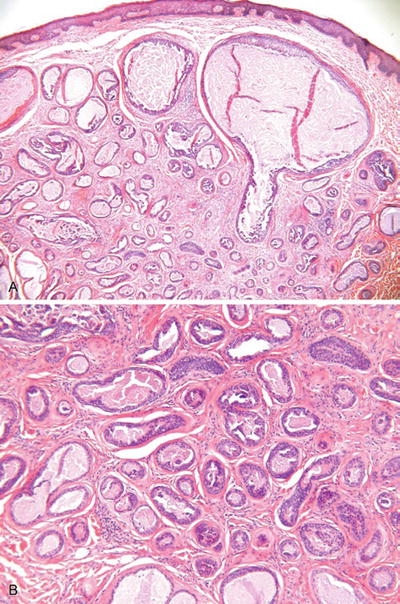
Fig. 18.27.
Tubular apocrine adenoma.
Clinical
♦
A well-defined nodule occurring most commonly on the scalp of adults
Microscopic
♦
An unencapsulated but well-demarcated proliferation of numerous ducts/glands lined by a bilayered to multilayered epithelium showing apocrine differentiation
♦
Small papillary structures and occasional cribriforming may be seen and resemble proliferative lesions in the breast
♦
These lesions may show some degree of nuclear atypia and mitotic activity but generally are not considered as carcinomas unless obvious infiltration of surrounding tissues is seen
Apocrine Carcinoma
Clinical
♦
A rare primary tumor of the skin that typically presents as an erythematous nodule with or without ulceration in older adults
♦
A variety of sites may be affected including the scalp, eye, ear, and anogenital regions among others
♦
Recurrences and metastases may occur
Microscopic
♦
A variety of histologic appearances may be present including cystic, papillary, sheetlike, and ductal variants
♦
Infiltration of adjacent tissues is seen, and pagetoidosis may be evident
♦
Nuclear pleomorphism may be mild to marked, and varying degrees of mitotic activity and necrosis may be evident
♦
By definition, areas of apocrine differentiation should be identified, at least focally
Differential Diagnosis
♦
Apocrine carcinoma of the breast has similar histologic and immunophenotypic characteristics and should be excluded clinically
Neuroendocrine-Derived Tumors
Merkel Cell Carcinoma (Fig. 18.28)
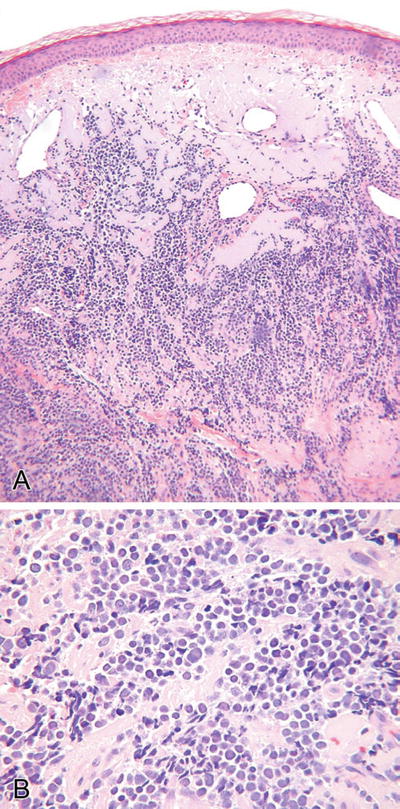
Fig. 18.28.
Merkel cell carcinoma (A, B).
Clinical
♦
An aggressive neoplasm typically presenting as a slowly growing nodule on the sun-exposed skin (head and neck region) of older adults
♦
The recurrence and metastatic rate is approximately 40–50%
Microscopic
♦
A variety of histologic forms may be seen and include sheetlike, ribboned, nested, trabecular, and organoid variants
♦
Pseudorosettes may be prominent
♦
These tumors are typically dermal based but frequently involve the subcutis and may show an intraepidermal growth pattern
♦
Focal areas of squamous, eccrine, or sebaceous differentiation may be seen, and these tumors may arise in conjunction with another histologically distinct neoplasm
♦
The cytologic and immunophenotypic appearance is characteristic and common to all variants
♦
Cytologically, the tumor cells have very high nuclear/cytoplasmic ratios, indistinct cell borders, and hyperchromatic and finely granular nuclei with inconspicuous nucleoli and thin nuclear membranes
♦
Nuclear molding and mitoses are abundant
♦
Immunophenotypically, the tumor cells express low molecular weight cytokeratin (CAM 5.2) in a perinuclear dot-like pattern which may also be seen with neurofilament staining
♦
Cytokeratin 20 staining is generally positive, and these tumors express a variety of neuroendocrine markers including neuron-specific enolase, chromogranin, synaptophysin, and neurofilament but are generally negative for S-100 protein and vimentin
Differential Diagnosis
♦
Merkel cell carcinomas must be differentiated from a variety of other neuroendocrine tumors of either metastatic or primary origin
♦
Small cell neuroendocrine carcinomas from visceral sites metastatic to skin have an essentially identical histologic appearance but have recently been reported to be cytokeratin 20 negative
♦
Cutaneous neuroblastoma generally shows a filamentous background and/or focal ganglion cell differentiation and is typically cytokeratin negative (except for the olfactory variant)
♦
Primitive neuroectodermal tumors/extraosseous Ewing sarcomas are usually cytokeratin negative and typically express MIC- 2
Soft Tissue Neoplasms and Developmental Anomalies
Adipocyte-Derived Tumors and Proliferations
Lipoma
Clinical
♦
A sporadic or multifocal tumor of the middle aged to elderly typically involving the trunk and/or extremities
Microscopic
♦
A thin encapsulated proliferation of mature adipose tissue
♦
The adipose tissue may be accompanied by a wide variety of other types of mesenchymal-derived tissue: fibrous (fibrolipoma), bone (osteolipoma), cartilage (chondroid lipoma), bone marrow (myelolipoma), mucoid substances (myxoid lipoma), smooth muscle (myolipoma), and smooth muscle and vessels (angiomyolipoma)
Angiolipoma
Clinical
♦
A painful, subcutaneous nodule(s) involving the upper extremities of young adults
Microscopic
♦
Thin encapsulated proliferations of mature adipocytes and variably sized, thin-walled vessels
♦
Microthrombi are readily identified within the vessel lumina
♦
A cellular variant showing numerous small vessels with few adipocytes exists and must be differentiated from other vascular tumors that lack the proliferation of mature adipocytes
Spindle Cell Lipoma
Clinical
♦
A solitary, painless, subcutaneous nodule with a predilection for the base of the neck of middle-aged to older adults
Microscopic
♦
An encapsulated proliferation of mature adipocytes and bland, bipolar spindle cells embedded in a myxoid matrix with collagen fibers
♦
The spindle cells may predominate and typically show bland, uniform features
♦
Lipoblasts and the plexiform capillary network of myxoid liposarcoma are absent
♦
Occasional cases show bizarre, multinucleated cells and merge with pleomorphic lipoma
Pleomorphic Lipoma
Clinical
♦
Similar to spindle cell lipoma
Microscopic
♦
Similar to spindle cell lipoma with the addition of numerous, multinucleated (floret cells) cells with hyperchromatic, peripherally situated nuclei
Lipoblastoma and Lipoblastomatosi s
Clinical
♦
These are tumors of infants and young children and typically present as painless masses on the extremities in a localized (lipoblastoma) or diffuse (lipoblastomatosis) fashion
♦
The latter may recur with incomplete excision
Microscopic
♦
Multilobular proliferations of immature and mature adipocytes embedded in a myxoid matrix and separated by thin fibrous septae
♦
The adipocytes may show a wide spectrum of differentiation from spindle cells to multivacuolated lipoblasts to mature, univacuolated adipocytes
♦
These lesions tend to mature histologically with time
Lipofibromatous Hamartoma of Nerve
Clinical
♦
A tumorlike condition that presents as a mass of the wrist and/or forearm
♦
Typically, these patients are young children, but adult presentations also occur
♦
Sensory defects, paresthesias, pain, and macrodactyly may be prominent
♦
Due to the intimate association of this proliferation with nerves, surgical excision is contraindicated and may lead to permanent sensorimotor impairment
Microscopic
♦
A proliferation of benign fibroadipose tissue is evident in and around nerve fibers which show secondary degeneration, atrophy, and fibrosis
Lipomatosis
Clinical
♦
Multiple clinical forms exist including a diffuse variant typically involving the extremities or trunk of young children, a symmetrical variant (Madelung disease) involving the neck region of middle-aged adult males as well as visceral and pelvic variants
Microscopic
♦
All are unencapsulated proliferations of mature adipose tissue involving the subcutis, skeletal muscle, and, occasionally, other structures
Nevus Lipomatosus Superficialis
Clinical
♦
A hamartomatous proliferation typically presenting as multiple, polypoid papules or plaques on the buttocks, posterior trunk, or thigh of children to young adults
Microscopic
♦
Small lobules of mature adipocytes are evident in the superficial and mid dermis and may be associated with keratin plugs and loss of adnexal structures
Hibernoma
Clinical
♦
A slow-growing, asymptomatic mass of the chest or upper back of young to middle-aged adults
♦
Other sites may also be affected
Microscopic
♦
An encapsulated, multilobular tumor composed of an admixture of multivacuolated and univacuolated adipocytes and large cells with eosinophilic cytoplasm and distinct cell membranes
Liposarcoma
Clinical
♦
These are rare tumors of the skin that generally present as slowly growing subcutaneous masses in older adults
Microscopic
♦
The myriad of histologic types of liposarcoma are addressed in detail elsewhere
♦
Generally, the liposarcomas involving the skin are of the well-differentiated (atypical lipoma) or myxoid types
♦
The former have lipoma-like, sclerosing, and spindle cell variants which may recur but typically do not metastasize
Neural-Derived Tumors and Proliferations
Neurofibroma
Clinical
♦
Solitary and multiple forms exist
♦
The solitary variant is typically a soft, polypoid, flesh-colored tumor occurring in adults
♦
The multiple or diffuse variant has a strong association with neurofibromatosis type 1 and may show extensive, cosmetically deforming lesions with a bag-of-worms appearance and feel
♦
The diffuse variant is seen more frequently in childhood and adolescence
♦
The diffuse variant has definite malignant potential, while the sporadic variant lacks this characteristic
Microscopic
♦
A variety of histologic subtypes exist, but all are characterized by a proliferation of wavy, pointed spindle cells embedded in a variably collagenous to myxoid matrix
♦
These tumors are unencapsulated but are generally well circumscribed
♦
They do not infrequently incorporate dermal adnexal structures, but direct adnexal invasion is rare
♦
Plexiform, diffuse, myxoid, and pacinian variants exist
♦
The plexiform variant is thought to be diagnostic of neurofibromatosis, while the diffuse variant may also be associated with this disease at an increased rate
Special Studies
♦
Neurofibromas are derived from nerve and, as such, demonstrate positive staining for axonal markers such as neurofilament and silver impregnation techniques
♦
Similar to other neural-derived tumors, these lesions are also positive for S-100 protein, CD57, and neuron-specific enolase
Schwannoma (Neurilemmoma, Fig. 18.29)
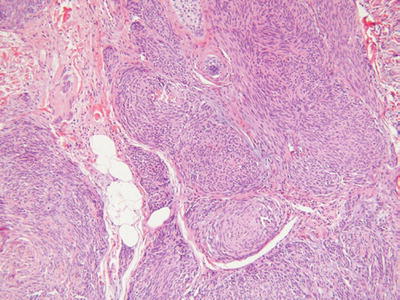
Fig. 18.29.
Plexiform schwannoma.
Clinical
♦
A peripheral nerve sheath-derived tumor that generally presents as a solitary nodule/mass on the head, neck, or extremities of adults
♦
Rarely, multiple tumors may be evident (schwannomatosis), and at least some of these cases are associated with neurofibromatosis type 2
♦
In general, schwannomas have little, if any, malignant potential
Microscopic
♦
A well-circumscribed, encapsulated tumor within the subcutis or deeper tissues
♦
Occasionally, dermal involvement may be evident
♦
The tumor is composed of spindle cells with wavy, pointed nuclei embedded in a collagenous and highly vascular stroma
♦
Classically, cellular, Antoni A areas with palisading, Verocay bodies are admixed with paucicellular, myxoid, Antoni B areas
♦
Degenerative changes are frequent findings and include hyalinization, vascular thrombosis, dystrophic mineralization, and hemorrhage
♦
The ancient variants typically show some degree of nuclear pleomorphism and enlargement with a smudgy chromatin pattern
♦
Plexiform variants exist and typically present in childhood or adolescence
♦
These tumors are composed of multiple, cellular, Antoni A-like, encapsulated nodules that must be differentiated from plexiform neurofibroma and plexiform fibrohistiocytic tumor
♦
Cellular variants are moderately to markedly cellular and may have mitotic activity. Nuclear pleomorphism and necrosis, however, are absent. Melanotic variants also exist and must be differentiated from malignant melanoma
Special Studies
♦
Schwannomas are derived from the peripheral nerve sheath and, therefore, lack axonal differentiation; that is, they are neurofilament and silver staining negative
♦
Schwann cells are S-100 protein positive and are generally surrounded by type IV collagen-rich basement membrane
♦
Epithelial membrane antigen typically stains the capsule of schwann omas
Traumatic Neuroma
Clinical
♦
Reactive, nonneoplastic proliferations of nerve in response to injury
♦
Typically present as small, often painful, nodules at sites of previous injury
Microscopic
♦
A well-localized but unencapsulated, haphazard proliferation of nerve fibers associated with dermal fibrosis (scar)
Palisaded and Encapsulated Neuroma (Solitary Circumscribed Neuroma, Fig. 18.30)
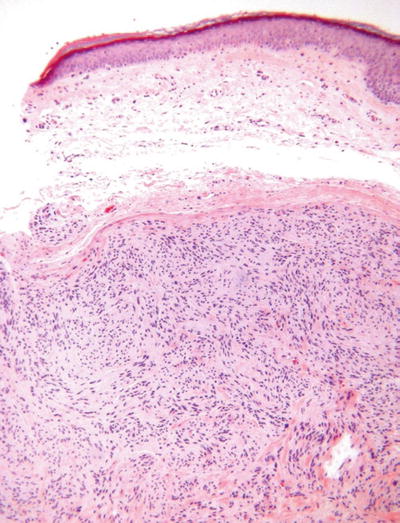
Fig. 18.30.
Palisaded and encapsulated neuroma.
Clinical
♦
Solitary, flesh-colored papule on the face of middle-aged to older adults
Microscopic
♦
A nodular to multinodular, often dumbbell-shaped proliferation of spindle cells embedded in a collagenous matrix
♦
These lesions are well circumscribed but only partially encapsulated
♦
The superficial component generally lacks a true capsule and tends to resemble a neurofibroma, while the deep component is encapsulated and resembles a schwannoma
♦
The spindle cells demonstrate wavy, pointed nuclei and lack mitotic activity
♦
Contrary to its name, true nuclear palisading is rare in this lesion
♦
A nerve may be evident entering the base of the lesion
Special Studies
♦
Like neurofibroma, this lesion contains both axons and schwann cells and, hence, is S-100 protein and neurofilament positive
Granular Cell Tumor (Fig. 18.31)
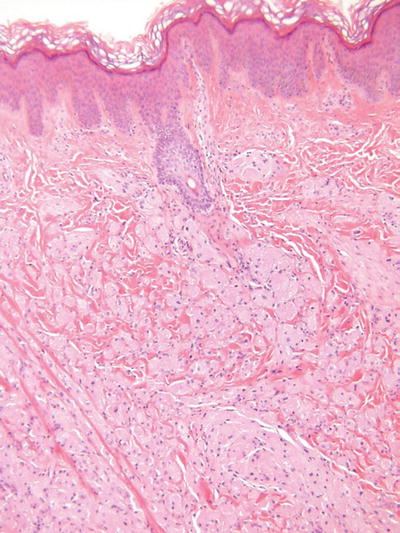
Fig. 18.31.
Granular cell tumor.
Clinical
♦
Slowly growing, sometimes painful, flesh-colored nodules with a predilection for the tongue, trunk, and extremities of adults
♦
While usually solitary, multiple, and familial variants exist
Microscopic
♦
Irregular fascicles and/or sheets of large, round to polygonal cells with eosinophilic, granular cytoplasm
♦
Cell borders are indistinct giving this tumor a syncytial appearance
♦
Nuclei are generally round to ovoid, central, and monomorphic
♦
Nuclear pleomorphism, mitotic activity, necrosis, and large tumor size may be associated with the rare malignant variants
♦
Prominent pseudoepitheliomatous hyperplasia of the epidermis is often seen overlying this tumor and must not be mistaken for squamous cell carcinoma
Special Studies
♦
While the cell of origin remains to be clarified, most authorities support a schwann cell derivation for these tumors
♦
Accordingly, these tumors are usually, but not always, S-100 protein and neuron-specific enolase positive
Differential Diagnosis
♦
Secondary granular cell change is not an uncommon finding in other tumor types (dermatofibroma, neurofibroma, etc.) which should be excluded prior to making the diagnosis of granular cell tumor
♦
This tumor should also be differentiated from congenital epulis discussed below
Congenital Epulis
Clinical
♦
A polypoid gingival lesion in newborns that may spontaneously regress
Microscopic
♦
Similar to granular cell tumor, but these lesions are S-100 protein negative
Nerve Sheath Myxoma (NSM ) and Cellular Neurothekeoma (Fig. 18.32)
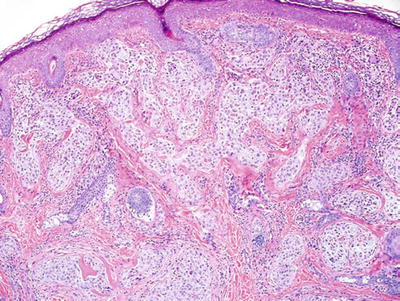
Fig. 18.32.
Neurothekeoma.
Clinical
♦
Soft, mobile, flesh-colored papules on the face or upper extremities of young adults
♦
These tumors may recur with incomplete excision
Microscopic
♦
NSM is a fascicular to lobular dermal tumor composed of spindle and stellate cells embedded in myxoid lobules which in turn are separated by fibrous septae
♦
Cellular neurothekeoma is composed of more uniform, epithelioid cells in nests and fascicles with minimal myxoid background material
♦
Mitotic activity and mild nuclear pleomorphism may be seen in both lesions
Special Studies
♦
NSM is typically S-100 protein+ and is likely derived from the peripheral nerve sheath
♦
Cellular neurothekeoma is S-100 protein− and its cell of origin is unclear
♦
The lack of S-100 protein positivity in the cellular variants allows these lesions to be readily distinguished from most melanocytic neoplasms
Perineurioma
Clinical
♦
A benign tumor of perineural origin that typically presents as a subcutaneous mass on the trunk and limbs of adults
Microscopic
♦
A well-circumscribed proliferation of bland spindle cells in fascicles with whorled and storiform areas
Special Studies
♦
Like perineural cells, this tumor is S-100 protein− and EMA+
Malignant Peripheral Nerve Sheath Tumor
General
♦
A rare, primary cutaneous malignancy frequently associated with neurofibromatosis type 1 when primary in the skin
♦
These lesions are discussed in detail in the chapter on soft tissue pathology
Cutaneous Meningothelial Heterotopias/Meningiomas (Fig. 18.33)
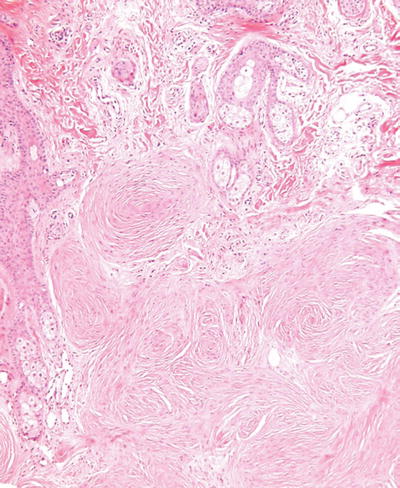
Fig. 18.33.
Meningioma.
Clinical
♦
Classic meningocele is typically a transilluminating mass along the lower spine and represents a congenital defect
♦
Rudimentary meningocele is thought to be a herniation of the meninges into the superficial tissues of the scalp with a subsequent loss of its intracranial attachment
♦
Cutaneous meningioma comes in three forms: type I is congenital lesion involving the head and paravertebral regions of children and is secondary to misplaced arachnoid cells during embryogenesis; type II occurs on the head and neck region of adults and is thought to be secondary to a proliferation of arachnoid cells through a cranial foramina; type III represents a metastasis or direct extension of tumor into skin from an intracranial primary lesion
Microscopic
♦
Meningoceles are typically cyst-like structures lined by arachnoid cells and having surrounding dense fibrous tissue with occasional collections of meningothelial cells in whorl-like structures
♦
Meningiomas are usually well-circumscribed deep dermal to subcutaneous proliferations composed of spindle cells arranged in fascicles and whorls with or without psammoma body formation
♦
Nuclear pleomorphism and mitotic activity may be seen, particularly in the type III variants
♦
Special studies
♦
Meningothelial proliferations are usually vimentin and EMA+
♦
Cytokeratin and S-100 protein may also be expressed by these tumors
Heterotopic Glial Tissue (Nasal Glioma)
Clinical
♦
Flesh-colored mass on the nasal bridge of infants to young adults. Intranasal involvement may also be present
♦
Radiographic studies should be performed to exclude an intracranial attachment
Microscopic
♦
Nodules of benign eosinophilic, fibrillar, glial tissue within the deep dermis and subcutis
♦
Rarely, neuronal cells may also be seen
Smooth Muscle-Derived Tumors and Proliferations
Smooth Muscle Hamartoma
Clinical
♦
A congenital, sometimes pigmented, indurated plaque on the trunk which typically presents in infancy
Microscopic
♦
A haphazard proliferation of smooth muscle fascicles in the dermis with or without basilar epidermal hyperpigmentation
Becker Nevus
Clinical
♦
An acquired, organoid, hyperpigmented plaque with hypertrichosis on the back of young adults and in adolescents
♦
This lesion may be associated with other congenital abnormalities
Microscopic
♦
Epidermal acanthosis and basilar hyperpigmentation occasionally associated with a mild haphazard proliferation of smooth muscle fascicles within the dermis
Piloleiomyoma (Pilar leiomyoma, Fig. 18.34)
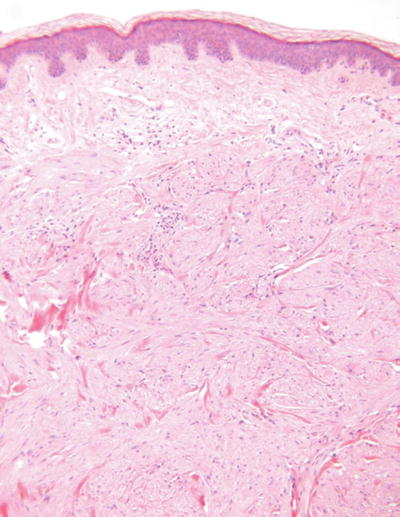
Fig. 18.34.
Piloleiomyoma.
Clinical
♦
Multiple, somewhat painful papules or nodules on the trunk or extremities of young adults
♦
May be inherited in an autosomal dominant fashion
Microscopic
♦
A fairly well-circumscribed yet irregular proliferation of smooth muscle fascicles within the dermis
♦
The individual cells have elongated eosinophilic cytoplasm and cigar-shaped nuclei
♦
No nuclear pleomorphism, mitotic activity, or necrosis is evident
Angioleiomyoma (Fig. 18.35)
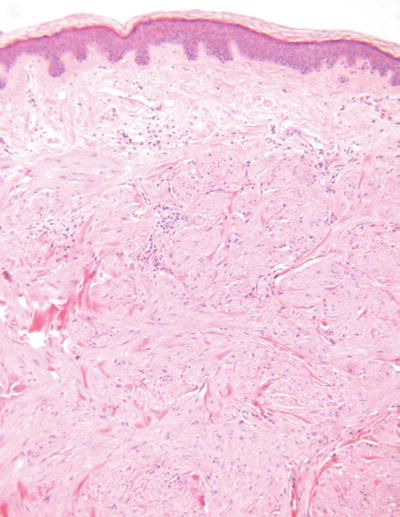
Fig. 18.35.
Angioleiomyoma.
Clinical
♦
A solitary, sometimes painful nodule on the extremities of adults
Microscopic
♦
A nodular, well-circumscribed proliferation of smooth muscle in fascicles admixed with numerous, variably sized vessels
♦
Degenerative changes are frequent
Leiomyosarcoma (Fig. 18.36)
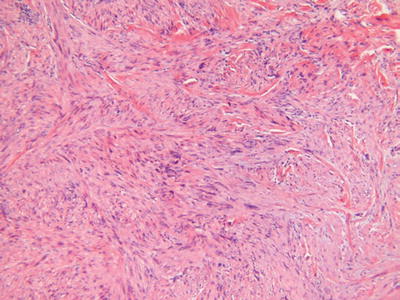
Fig. 18.36.
Leiomyosar coma.
Clinical
♦
Two clinical variants exist: a superficial or cutaneous variant and a deep or subcutaneous variant
♦
The former predominates on the limbs of young adults and is likely derived from the arrector pili muscle
♦
This variant may locally recur but generally does not metastasize
♦
The subcutaneous variant predominates on the limbs of the elderly and has both local recurrence and metastatic potential
Microscopic
♦
The cutaneous variant demonstrates an irregular, infiltrating, and haphazard proliferation of smooth muscle bundles reminiscent of piloleiomyoma
♦
However, unlike piloleiomyoma, this tumor has low-grade nuclear pleomorphism, mitotic activity, and, rarely, necrosis
♦
The deep or subcutaneous variant is akin to leiomyosarcomas arising elsewhere and consists of a nodular, at least focally infiltrating, proliferation of smooth muscle fibers in well-formed to ill-defined bundles with varying degrees of nuclear pleomorphism, mitotic activity, and necrosis
Fibrohistiocytic, Histiocytic, and Langerhans Cell-Derived Proliferations
Fibrous Histiocytoma (Dermatofibroma, Fig. 18.37)
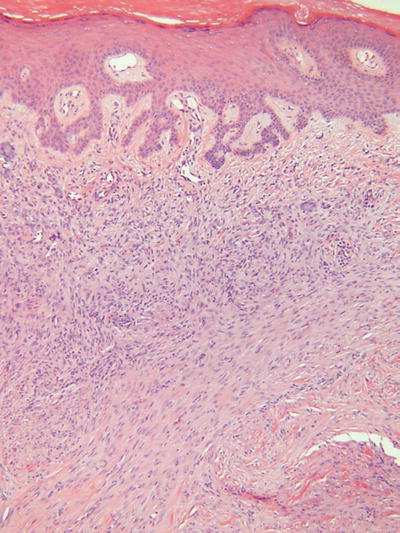
Fig. 18.37.
Dermatofibroma.
Clinical
♦
Usually single, occasionally multiple, slightly elevated, smooth, flesh-colored to hyperpigmented nodules on the extremities or trunk of adults
Microscopic
♦
Numerous subtypes have been described, but all are generally made up of a proliferation of histiocytes, fibroblasts, and collagenous tissue in varying proportions
♦
The prototypical lesion (dermatofibroma) consists of a fairly well-circumscribed, unencapsulated, middermal proliferation with feathery edges, an overlying Grenz zone, and epidermal hyperplasia with basilar hyperpigmentation
♦
The cellular component consists of spindled, fibroblast-like cells admixed with plump histiocytic cells in an irregular fashion
♦
Storiform areas may be evident, and there may be focal extension into the subcutis
♦
Chronic inflammatory cells, multinucleated giant cells, xanthomatized histiocytes, and hemosiderin-laden histiocytes are also frequently identified within these lesions
♦
Important variants include atypical dermatofibroma (dermatofibroma with monster cells), aneurysmal fibrous histiocytoma, and epithelioid cell histiocytoma, all of which may be mistaken for other more aggressive entities
♦
Atypical dermatofibroma is a lesion showing large, hyperchromatic, multinucleated giant cells in addition to typical dermatofibroma features and should be distinguished from atypical fibroxanthoma and malignant fibrous histiocytoma
♦
Aneurysmal fibrous histiocytoma is a fibrous histiocytoma with prominent intralesional hemorrhage and cystic, pseudovascular spaces
♦
This lesion should be distinguished from angiomatoid fibrous histiocytoma, a lesion of intermediate-grade malignancy occurring in the pediatric population
♦
Epithelioid cell histiocytoma is composed of a polypoid well-circumscribed proliferation of angulated, epithelioid, histiocytic-appearing cells surrounded by an epidermal collarette
♦
This lesion should be distinguished from melanocytic tumors, both Spitz nevus and melanoma
Special Studies
♦
Most fibrous histiocytomas express FXIIIa but are negative for CD34
♦
Dermatofibroma sarcoma protuberans tends to have the opposite staining pattern, but overlap and divergent staining patterns occasionally occu r
Angiomatoid Fibrous Histiocytoma (Fig. 18.38)
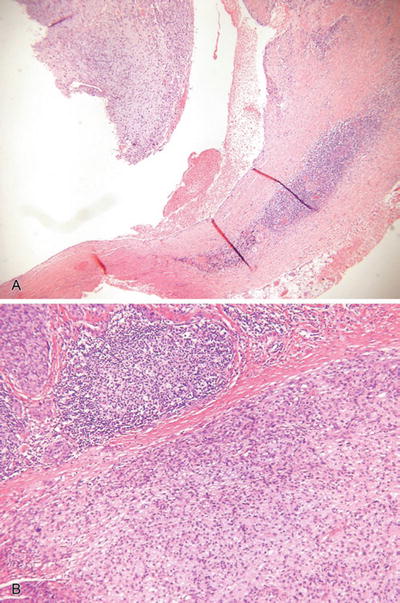
Fig. 18.38.
Angiomatoid fibrous histiocytoma (A, B).
Clinical
♦
A fairly deep-seated, usually subcutaneous, nodule or mass within the extremities of children and adolescents
♦
This lesion is considered to be of borderline or intermediate malignancy and may recur and, rarely, metastasize
Microscopic
♦
A circumscribed, partially cystic and lobular mass usually centered on subcutaneous tissue
♦
This tumor is composed of an admixture of cystic, blood-filled pseudovascular spaces, myxoid lobules of histiocytic-appearing cells, and peripheral lymphocytic inflammation and fibrosis
♦
The neoplastic cells are somewhat spindled to plump, epithelioid cells with eosinophilic to amphophilic cytoplasm and mildly pleomorphic nuclei
♦
Mitotic figures may be evident
Special Studies
♦
The histiocytic-appearing cells often show expression of smooth muscle actin, desmin, and CD34, suggesting to som e a myofibroblastic derivation to this tumor
Plexiform Fibrohistiocytic Tumor
Clinical
♦
A slowly growing deep dermal to subcutaneous mass on the extremities of children to young adults
♦
This is a tumor of intermediate malignancy that may recur and rarely metastasizes
Microscopic
♦
An unencapsulated, irregular biphasic tumor consisting of short fibromatosis-like fascicles of plump spindle cells admixed with nodules of histiocytic and osteoclast-like giant cells with minimal nuclear pleomorphism
♦
The admixture of nodules and fascicles gives this tumor a plexiform appearance at low power
Juvenile Xanthogranuloma (Fig. 18.39)
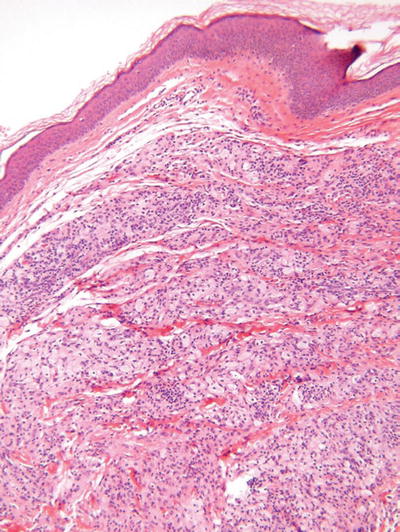
Fig. 18.39.
Juvenile xanthogranuloma.
Clinical
♦
Solitary to multiple, yellow to red papules on the head and neck region of infants are the most common presentation
♦
However, adolescents and adults may also be affected, and other body sites may be involved
♦
Visceral involvement may be evident with the eye being the most common extracutaneous site of involvement
♦
Typically, the cutaneous lesions regress over time
Microscopic
♦
Fairly well-circumscribed collection of histiocytes within the dermis admixed with Touton giant cells, xanthomatized histiocytes, and chronic inflammatory cells
♦
The epidermis is spared, but periadnexal involvement is common
♦
Spindle cell and inflammatory cell-rich variants exist
Reticulohistiocytoma and Multicentric Reticulohistiocytosis
Clinical
♦
Solitary and multicentric variants exist
♦
The former is usually a yellow to brown nodule on the upper body of adults, while the latter shows multiple lesions associated with a destructive arthritis and constitutional symptoms, also in adults
♦
Occasionally, the multicentric variant is associated with visceral neoplasia
Microscopic
♦
Both variants are characterized by fairly well-circumscribed proliferations of large, eosinophilic histiocytes with “glassy” cytoplasm
♦
The individual cells may contain more than a single nucleus, but Touton giant cells are usually absent, and xanthomatized histiocytes are not generally present
♦
Mild nuclear pleomorphism and admixed inflammation with eosinophils and lymphocytes may be evident
Atypical Fibroxanthoma (Fig. 18.40)
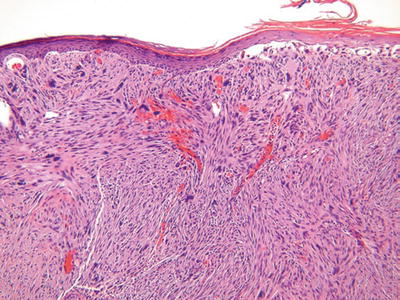
Fig. 18.40.
Atypical fibroxanthoma.
Clinical
♦
Typically a solitary, often ulcerated polypoid nodule on the sun-exposed skin (head and neck) of the elderly
♦
A less common variant occurs on the trunk and extremities of young adults
♦
These tumors, when limited to superficial tissues, may locally recur but have minimal metastatic potential
Microscopic
♦
A nodular, often well-circumscribed, proliferation of very pleomorphic epithelioid to spindle cells with frequent mitoses, often atypical
♦
These tumors are usually centered on the dermis and frequently about the dermal–epidermal junction where they stop abruptly
♦
Epidermal ulceration is common, but true epidermal involvement with pagetoid spread should raise concerns about malignant melanoma or squamous cell carcinoma
♦
Rarely, the tumor is composed of spindle cells in poorly defined fascicles and may be mistaken for leiomyosarcoma
♦
While occasional tumors may show superficial involvement of the subcutaneous tissues, extensive subcutaneous involvement and/or prominent vascular invasion and necrosis should lead to an alternate diagnosis (e.g., malignant fibrous histiocytoma)
Special Studies
♦
This tumor is a diagnosis of exclusion; therefore, S-100 protein, cytokeratin, and desmin should be negative by immunoperoxidase technique to exclude malignant melanoma, carcinoma, and leiomyosarcoma, respectively
♦
Smooth muscle actin may be expressed by a subset of these tumors and is not indicative of smooth muscle differentiation (leiomyosarcoma) in the absence of desmin positiv ity
Malignant Fibrous Histiocytoma
General
♦
These tumors are rarely primary cutaneous lesions and have overlapping histologic features with atypical fibroxanthoma
♦
The latter tumor is often considered to be a superficial variant of malignant fibrous histiocytoma with minimal metastatic potential
♦
The term malignant fibrous histiocytoma should be used for those tumors that demonstrate deep tissue involvement, vascular invasion, or extensive necrosis as detailed above
♦
The histologic subtypes and clinical distribution of this tumor are detailed in the chapter on soft tissue tumor
Xanthomas and Xanthelasma (Fig. 18.41)
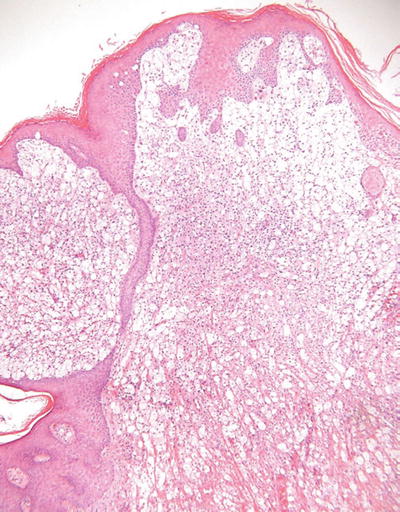
Fig. 18.41.
Verruciform xanthoma.
Clinical
♦
Numerous clinical and histologic subtypes exist, many of which are related to systemic lipid abnormalities and represent storage disorders
♦
Eruptive
Yellow papules on the buttocks and other sites associated with type I hyperlipoproteinemia
♦
Tuberous
Yellow papules and nodules on the extensor surfaces associated with hyperlipoproteinemia types II–IV



