Fig. 45.1.
F ocal nodular hyperplasia consisting of a well-circumscribed, lobulated lesion with a central stellate scar arising in a noncirrhotic liver.
The scar is eccentrically located in small, early lesions but assumes a central location as the lesion enlarges
Microscopic
♦
The typical feature is the presence of abnormal vessels with eccentrically thickened walls within the radial scar and the fibrous septa (Fig. 45.2)
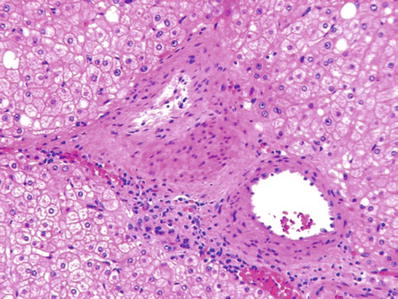

Fig. 45.2.
C entral scar of focal nodular hyperplasia showing atypical vessels with eccentrically thickened walls.
Bile ductules and a mild inflammatory infiltrate are present within the fibrous septa
No bile ducts are present within the lesion
♦
Proliferative nodules of hepatic parenchyma consist of crowded hepatocytes which are of similar or smaller size than normal hepatocytes
These hepatocytes often contain medium or large droplet fat
Special Stains
♦
Immunohistochemical stain for glutamine synthetase shows a characteristic pattern of irregularly shaped geographic areas of staining (“map-like” pattern)
♦
Immunohistochemistry for β-catenin shows membranous staining
There is no nuclear staining
Differential Diagnosis
♦
Hepatocellular adenoma (HCA)
Hepatocellular Adenoma
Clinical
♦
HCA occurs most commonly in women of reproductive age taking oral contraceptive steroids with a causal link related to dose, duration of use, and patient’s age
There is an absolute risk of <3 per 100,000 oral contraceptive users per year for women under 30 years of age
♦
HCA also occurs in adult men and children and is associated with a variety of other factors such as anabolic/androgenic steroids, glycogenosis type 1, familial adenomatous polyposis (FAP), maturity-onset diabetes of the young (MODY3), and obesity
♦
There are four types of HCA with distinct clinical, morphological, and molecular characteristics (Table 45.1)
Table 45.1.
Molecular Subtypes of Hepatocellular Adenoma (HCA)
Subtype | Molecular abnormalities | Distinctive microscopic features | Distinctive IHC features | Associations |
|---|---|---|---|---|
HCA with mutations in HNF1A (30–40%) | Biallelic inactivation of HNF1A; both somatic (90%) or one germline and one somatic (10%) | Marked steatosis | L-FABP negative | Women; Germline mutations associated with MODY3 (HNF1A) FAP |
HCA with mutations in β-catenin (10–15%) | Mutation in β-catenin gene (CTNNB1) | Cytological atypia Pseudoglandular pattern | Aberrant nuclear positivity for β-catenin in a heterogenous distribution | Androgen intake Glycogenosis type 1 FAP |
Inflammatory/telangiectatic HCA (40–50%) | In-frame deletions of gp130 (IL6ST) (60%) | Inflammatory infiltrates, sinusoidal dilatation, telangiectasia | SAA and CRP positive | Obesity, alcohol intake, inflammatory syndrome with increased serum levels of CRP |
HCA, not otherwise specified (10%) | None defined | Negative for steatosis, cytological abnormalities, inflammatory infiltrates | Normal L-FABP positivity, no other specific findings | None defined |
♦
Spontaneous regression after discontinuation of drug use is rare, as are instances of malignant transformation
Malignant transformation is most likely to occur in the subgroup of tumors with β-catenin mutations (Table 45.1)
♦
Patients may be asymptomatic or present with episodic right upper quadrant pain and discomfort
Rupture may lead to severe abdominal pain, hemorrhage, and shock
♦
The tumor does not produce α-fetoprotein and elevated levels are not found in serum
♦
Treatment consists of surgical resection
Macroscopic
♦
Usually solitary and may be pedunculated
The tumors may become very large and assume sizes up to 30 cm
The tumor appears as a bulging mass with dilated blood vessels coursing on its surface
♦
On cut surface, the tumor appears soft, well demarcated, but unencapsulated
They are usually yellow to tan brown in color and frequently show areas of hemorrhage
Tumors associated with HNF1A mutations are steatotic and appear yellow in color, whereas the telangiectatic/inflammatory subtype appears dar k brown
♦
HCA are not associated with cirrhosis and the remaining liver is usually normal
Microscopic
♦
Made up purely of hepatocytes arranged in two–three-cell-thick plates
Hepatic plates are never more than three cells thick
Hepatic plates are separated by endothelial-lined sinusoids, which may not be conspicuous (Figs. 45.3 and 45.4)
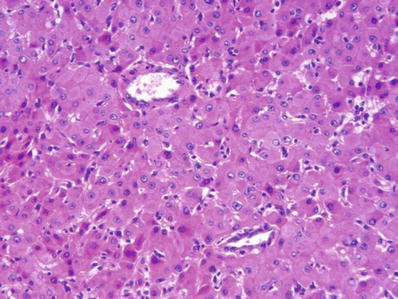
Fig. 45.3.
Hep atocellular adenoma showing hepatocytes arranged as two or three-cell-thick plates with thick-walled arterioles scattered within the lesion. The tumor cells are slightly larger than normal hepatocytes and have regular nuclei.
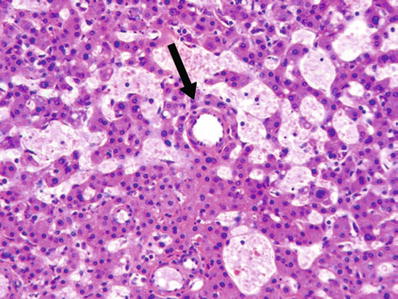
Fig. 45.4.
Areas of sinusoidal dilatation are commonly seen within hepatocellular adenomas. Arrow points to a thick-walled arteriole.
Stroma is rich in reticulin fibers which surround individual hepatocytes (Fig. 45.5)
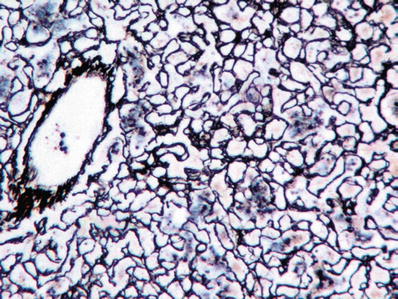
Fig. 45.5.
Reticulin stain of a hepatocellular adenoma shows reticulin fibers around individual hepatocytes.
Pseudoacinar (pseudoglandular) structures may be seen in CTNNB1-mutated HCA
♦
Tumor cells are generally larger than hepatocytes and have uniform nuclei
Nuclear pleomorphism may be seen in CTNNB1-mutated HCA
Cytoplasm may be pale or clear
♦
Steatosis is a common finding in adenomas with HNF1A mutations
Mitoses are absent in HCA
♦
Bile may be present as cytoplasmic droplets or plugs in distended canaliculi
♦
♦
Sinusoidal dilatation and peliosis hepatis are common findings (Fig. 45.4)
Areas of telangiectasia are particularly common in the telangiectatic/inflammatory subtype
♦
Rare features include epithelioid and giant cell granulomas, steatohepatitis, and extramedullary hematopoiesis
Immunohistochemistry
♦
HCA with mutations in HNF1A show loss of staining for liver-type fatty acid-binding protein (L-FABP)
♦
HCA with mutations in CTNNB1 show aberrant nuclear positivity for β-catenin in addition to usual membranous positivity, normal positivity for L-FABP, and positivity for glutamine synthetas e
♦
Telangiectatic/inflammatory HCA show staining with serum amyloid A (SAA) and C-reactive protein (CRP) and normal L-FABP positivity
Differential Diagnosis
♦
Focal nodular hyperplasia
♦
Well-differentiated HCC
♦
HCC arising in HCA
Premalignant and Malignant
Dysplastic Foci
Clinical
♦
Dysplastic foci are recognized microscopically within the liver parenchyma in chronic liver diseases, particularly in cirrhosis
Microscopic
♦
Dysplastic foci consist of clusters of dysplastic hepatocytes, less than 1 mm in size
♦
Depending on the cell type, dysplastic foci are classified into small cell type or large cell type
Small cell dysplastic foci are characterized by overall increased cellularity due to decreased cytoplasmic volume and increased nuclear/cytoplasmic ratio with mild nuclear pleomorphism and hyperchromasia
♦
Large cell foci are characterized by increased volume of both cytoplasm and nucleus with pleomorphism and hyperchromasia
Dysplastic Nodule
Clinical
♦
A dysplastic nodule may be radiographically detectable within the liver parenchyma in cirrhosis
Macroscopic
♦
A distinctive nodule that bulges on the cut surface of the liver
♦
Range in size from 1 to 2 cm
Microscopic
♦
Histologically subclassified into low-grade or high-grade nodule
♦
A low-grade dysplastic nodule is characterized by a “clonal” appearance of the hepatocytes with no or minimal cytologic atypia
Hepatic trabeculae are one to two cells thick
♦
A high-grade dysplastic nodule is characterized by two–three-cell-thick liver plates with a certain degree of cytological atypia and architectural abnormality, which are, however, insufficient for a diagnosis of HCC
Differential Diagnosis
♦
Large regenerative nodule
♦
Well-differentiated HCC
Hepatocellular Carcinoma
Clinical
♦
HCC is globally the fifth most common malignancy and the third leading cause of death
♦
Most HCCs develop in a background of cirrhosis of varied etiology
♦
The incidence of HCC varies widely around the world reflecting the varying incidence of the underlying risk factors
The incidence is highest in Africa and Southeast Asia due to the high incidence of hepatitis B virus (HBV) infection
The incidence is lower in Europe, North and South America, and Australia
Japan, North Africa, and the Middle East constitute areas of intermediate risk
♦
The etiologic factors underlying the development of cirrhosis may impart additional and independent risk for HCC
HBV DNA integrates into the host genome and transactivates oncogenes
Aflatoxin B1 causes a specific change (molecular “fingerprint”) in the TP53 gene (G > T at codon 249)
α-1 antitrypsin and tyrosinemia confer a risk greater than what can be ascribed to cirrhosis alone
♦
There is a weak association with xenobiotics such as organochlorine pesticides, polychlorinated biphenyls, thorotrast, and vinyl chloride
♦
Clinical presentation includes:
Middle-aged and older patients with long-standing cirrhosis and rapidly developing liver failure (low-incidence areas)
Young adults presenting with pain, weight loss, or hemorrhage (high-incidence areas)
♦
Significantly elevated serum α-fetoprotein may be diagnostic but is only moderately sensitive
Imaging studies have better sensitivity but may miss small lesions, especially in a cirrhotic background
♦
Lymph node metastases are common and treatment results are poor
♦
Most patients die within months of presentation, although better results are seen with small and incidental tumors
Small (<2 cm) and early HCCs comprise 15% of all liver cell cancers from Southeast Asia
♦
These have a better prognosis because they have not yet attained an angioinvasive phenotype
Macroscopic
♦
Most HCCs arise in a background of cirrhosis (Fig. 45.6)
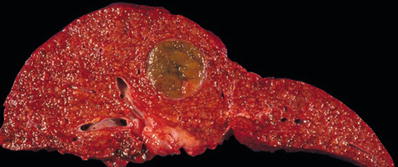

Fig. 45.6.
Hepatocel lular carcinoma seen as a soft green-yellow nodule arising in a cirrhotic liver.
♦
HCC appears as a soft, yellow-green, or reddish mass of varying size
♦
Satellite nodules are common and are defined as smaller tumor nodules situated <1 cm around a large mass
♦
Tumor has a high propensity to invade into portal veins
Microscopic
♦
Most characteristic pattern recapitulates normal liver architecture with trabeculae and intervening sinusoidal vascular channels (Figs. 45.7 and 45.8)
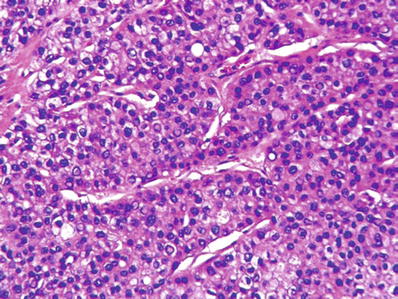
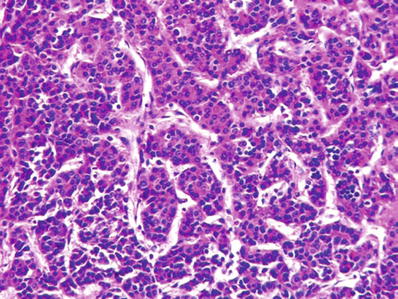

Fig. 45.7.
Hepatocellular carcinoma showing a macrotrabecular pattern consisting of hepatocytes arranged as thick trabecula separated by sinusoids.

Fig. 45.8.
Hepatocellular carcinoma showing a microtrabecular pattern consisting of slender trabecula separated by sinusoids.
♦
Tumor cells are large with abundant cytoplasm, high nuclear/cytoplasmic ratio, and large nucleoli
They have slightly more basophilic cytoplasm than normal liver cells and prominent nucleoli
Intracellular inclusions are common and include eosinophilic inclusions of α-1 antitrypsin and pale bodies, some of which stain for fibrinogen
Mallory hyaline and fat droplets may be present
♦
The presence of bile is diagnostic of HCC
♦
Several histologic patterns may occur, usually in combination with each other
Major histological patterns include trabecular, pseudoglandular, and solid growth (Figs. 45.9 and 45.10)
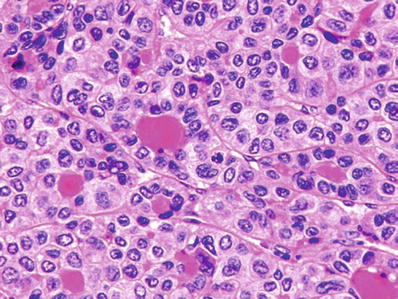
Fig. 45.9.
Hepatocellular carcinoma showing pseudoglandular pattern with eosinophilic secretions within the gland-like structures.
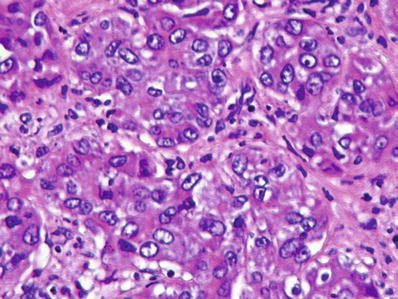
Fig. 45.10.
Poorly differentiated hepatocellular carcinoma growing in a solid pattern.
Less common patterns include steatohepatitic, inflammatory, anaplastic giant cell; clear cell; and spindle cell (Figs. 45.11, 45.12, 45.13, and 45.14)
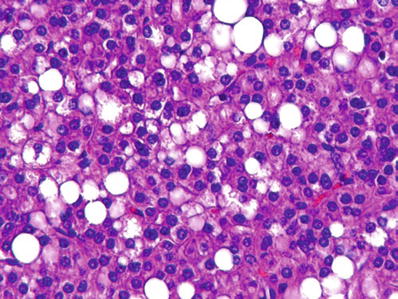
Fig. 45.11.
Hepatocellular carcinoma showing macrovesicular fatty change.
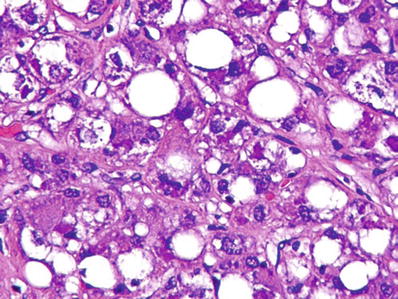
Fig. 45.12.
Steatohepatitic hepatocellular carcinoma with fatty change and Mallory hyaline.
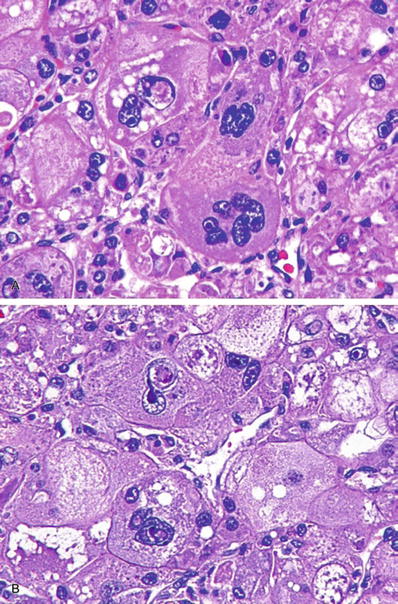
Fig. 45.13.
Hepatocellular carcinoma with bizarre, multinucleated giant tumor cells.
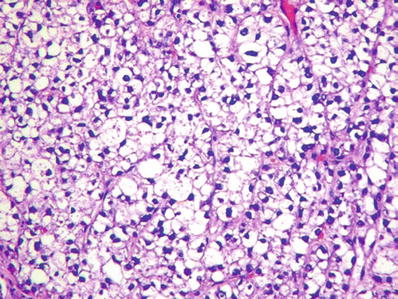
Fig. 45.14.
Hepatocellular carcinoma with a clear cell pattern.
The significance of these variant patterns is not well defined
♦
Diagnosis on fine needle aspiration cytology is made by the presence of endothelial cells around clusters of malignant cells and the presence of bile (Figs. 45.15 and 45.16)
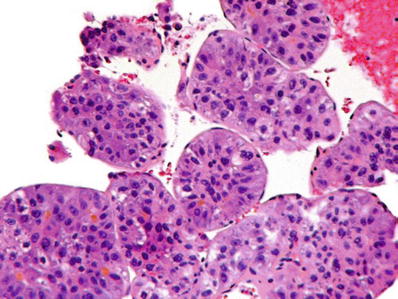
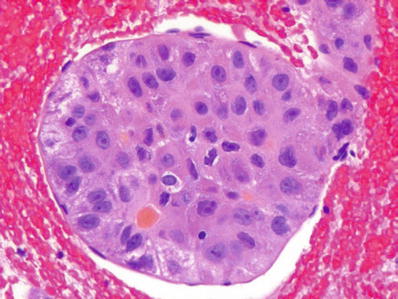

Fig. 45.15.
Hepatocellular carcinoma from a fine needle aspiration specimen showing the characteristic covering of tumor groups by an endothelial lining.

Fig. 45.16.
Hepatocellular carcinoma showing the presence of bile which is a definitive diagnostic feature.
Special Stains
♦
A reticulin stain shows thick trabecula of hepatocytes distinguishing HCC from an HCA or regenerative nodule which show two–three-cell-thick plates (Fig. 45.17)
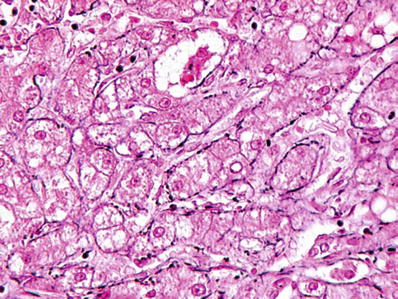

Fig. 45.17.
Reticulin staining of hepatocellular carcinoma shows the tumor to be composed of thick trabecula.
♦
A mucin stain is negative in HCC distinguishing it from metastatic adenocarcinoma
Immunohistochemistry
♦
HCC is positive for HepPar-1 (also known as hepatocyte-specific antigen), glypican-3, and arginase-1
♦
Polyclonal CEA and CD10 highlight the bile canaliculi (canalicular pattern of staining)
♦
α-Fetoprotein is positive in approximately 25% of HCC
♦
Cytokeratin 7 is positive in approximately 20% of HCC
This is associated with worse prognosis
Variants
♦
Scirrhous-type HCC is negative for HepPar-1 and frequently positive for adenocarcinoma-related markers, which can lead to an erroneous diagnosis of adenocarcinoma. Glypican 3 and arginase are the most reliable markers in this setting
♦
Chromophobe HCC with abrupt anaplasia is characterized by smooth chromophobic cytoplasm, abrupt focal nuclear anaplasia, and scattered microscopic pseudocysts
This variant has a distinctive molecular phenotype, namely, alternative lengthening of telomere (ALT) detectable by FISH
ALT is a telomerase independent mechanism of telomere maintenance
♦
Rarely, HCC may be pedunculated
This variant has a better prognosis if the tumor is localized and surgically resectable
Differential Diagnosis
♦
HCA
♦
Metastatic tumors
♦
Mixed hepatocellular cholangiocarcinoma
Fibrolamellar Carcinoma
Clinical
♦
Fibrolamellar ca rcinoma (FLC) occurs in patients between the ages of 18 and 45 years
♦
Patients do not have cirrhosis, and hepatitis B infection is infrequent
♦
Patients present with abdominal pain, malaise, and weight loss
A mass is usually readily palpable
♦
Has a better prognosis than usual HCC
This is related to early stage at presentation when the tumor is localized to the liver and often resectable
Stage for stage; however, the prognosis is the same as for usual HCC
The 5-year survival rate is 10–50%
Macroscopic
♦
Appears as a well-defined, solitary mass
Mass has a lobulated appearance with a central scar and interconnecting fibrous bands
Small satellite nodules may be present around the main mass
♦
Metastases to regional lymph nodes and lungs may be present
Microscopic
♦
Shows a characteristic microscopic appearance of sheets of hepatocytes separated by lamella of dense, hyalinized collagen (Figs. 45.18 and 45.19)
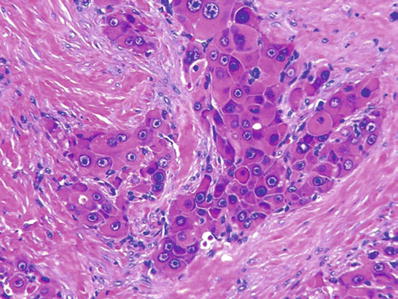
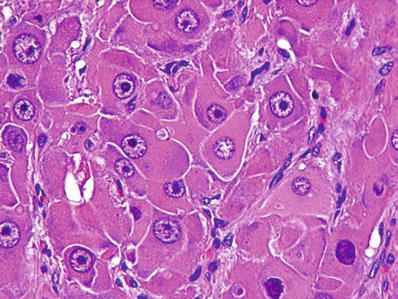

Fig. 45.18.
Fibrolamellar carcinoma consists of sheets of tumor cells separated by lamellar fibrosis.

Fig. 45.19.
Fibrolamellar carcinoma consists of large tumor cells with abundant eosinophilic cytoplasm and large eosinophilic nucleoli.
Tumor cells are large and contain abundant granular, eosinophilic cytoplasm with central nuclei containing prominent eosinophilic central nucleoli
Granular, eosinophilic cytoplasm corresponds to abundant mitochondria
Numerous intracytoplasmic inclusions are present
These correspond to eosinophilic globules of α-1 antitrypsin and “pale bodies,” some of which stain for fibrinogen (Figs. 45.20 and 45.21)
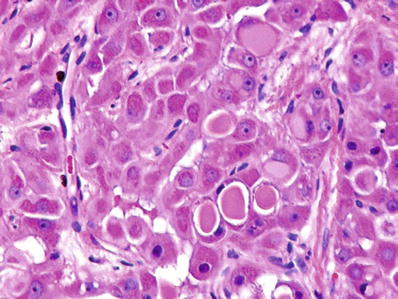
Fig. 45.20.
Fibrolamellar carcinoma with intracytoplasmic eosinophilic inclusions of α-1 antitrypsin.
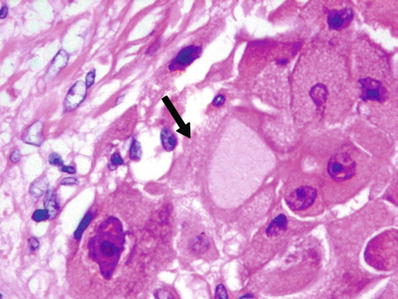
Fig. 45.21.
Fibrolamellar carcinoma with pale body (arrow).
♦
Bile production may be seen which confirms hepatocellular differentiation
♦
Fat droplets may be occasionally present
Immunohistochemistry
♦
Polyclonal CEA and CD10 outline the bile canaliculi
♦
Cytokeratin 7 is positive in most cases
♦
α-Fetoprotein is usually negative
Hepatoblastoma
Clinical
♦
Hepatoblastoma is a tumor of young children
The majority of cases occur in children younger than 5 years of age
The peak incidence is in the first 2 years of life
Rare cases have been reported in adolescents and young adults
♦
The relative incidence of childhood tumors is fairly identical worldwide
Nephroblastoma/neuroblastoma/hepatoblastoma = 6:3:1
♦
There is a significant association with familial adenomatous polyposis, nephroblastoma, Down syndrome, prematurity, and low birth weight
♦
The Wnt/β-catenin pathway is most commonly affected in hepatoblastoma
Approximately 70% of sporadic hepatoblastomas show abnormal accumulation of β-catenin
A common deletion of exon 4 of the CTNNB1 gene is detected in 80–90% of hepatoblastomas
♦
Patients present with failure to thrive, weight loss, and rapidly enlarging upper abdominal mass with markedly elevated serum levels of α-fetoprotein
♦
Prognosis depends on the ability to completely resect the tumor
Since most tumors are large and unresectable at presentation, preoperative chemotherapy is commonly employed to decrease size and allow resection
Macroscopic
♦
Appears as a single, large mass up to 25 cm in size
♦
Cut surface is variegated with areas of necrosis, cystic degeneration, and hemorrhage
♦
Background liver is noncirrhotic
Microscopic
♦
May be either epithelial or mixed epithelial–mesenchymal in type (Box 45.1)
Four epithelial variants are described:
Fetal (Figs. 45.22 and 45.23)
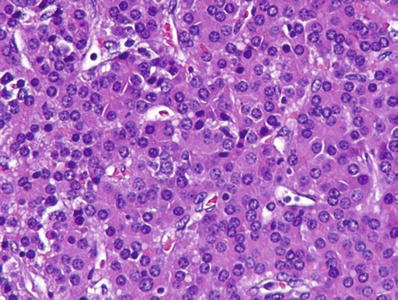
Fig. 45.22.
Fetal hepatoblastoma showing cells with slightly increased nuclear/cytoplasmic ratio, eosinophilic cytoplasm, and central, round, regular nuclei.
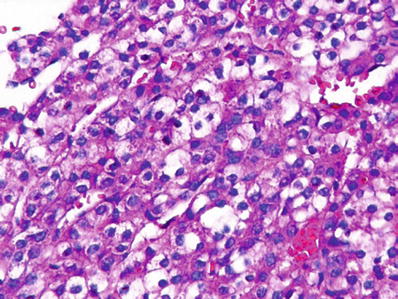
Fig. 45.23.
Fetal areas of hepatoblastoma often consist of cells with clear cytoplasm.
Embryonal (Figs. 45.24 and 45.25)
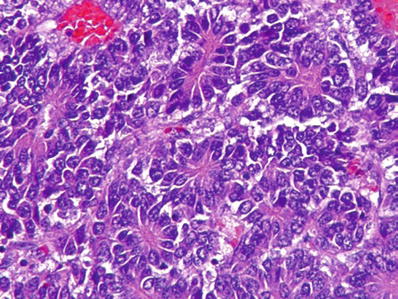
Fig. 45.24.
Hepatoblastoma showing areas that resemble embryonal hepatoblasts with pseudorosetting.
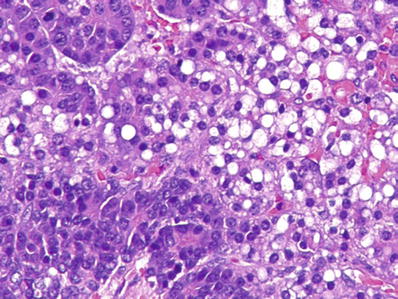
Fig. 45.25.
Hepatoblastoma with admixture of fetal and embryonal pattern.
Small cell undifferentiate d (SCUD) (Fig. 45.26)
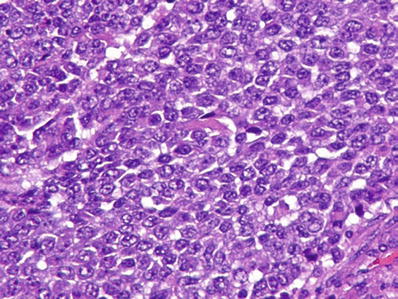
Fig. 45.26.
Hepatoblastoma with anaplastic (small cell) component.
♦
Macrotrabecular
Epithelial hepatoblastoma tries to recapitulate the developing liver
Mesenchymal elements include the bone, cartilage, squamous epithelium, glandular epithelium, undifferentiated mesenchyme, and muscle (Fig. 45.27)
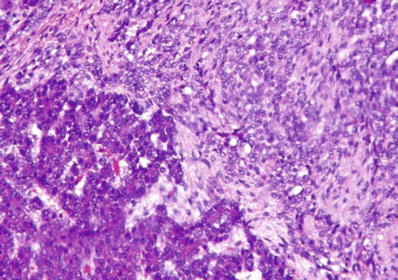
Fig. 45.27.
Hepatoblastoma with admixture of epithelial and mesenchymal elements; the latter is seen as an undifferentiated spindle cell component.
♦
Various elements are present together in variable combinations
Fetal and embryonal el ements are invariably present in most tumors in varying proportions
Mixed fetal–embryonal hepatoblastoma represents the most common variant of hepatoblastoma (Fig. 45.25)
♦
Fetal hepatoblastoma consists of large, polygonal fetal-type cells with oval nuclei, single nucleoli, and granular or clear cytoplasm organized into irregular plates with bile canaliculi and sinusoids (Figs. 45.22 and 45.23)
Islands of extramedullary hematopoiesis are commonly seen in fetal hepatoblastoma
♦
♦
Small cell undifferentiated hepatoblastoma consists of “small blue cells” with a high nuclear/cytoplasmic ratio, scant cytoplasm, and no nucleoli (Fig. 45.26)
The cells are arranged as sheets or clusters of cells
Mitoses are frequent
♦
The macrotrabecular pattern of hepatoblastoma resembles HCC
The macrotrabecular variant is morphologically and clinically not well defined
This is a controversial variant and increasingly believed to represent HCC
♦
Histological types with prognostic significance include:
The purely fetal variant which has a better prognosis
SCUD which has a bad prognosis even when present in small foci
SCUD is not infrequent and extensive sampling is required to exclude its presence due to the adverse prognostic implications
The macrotrabecular variant which has a bad prognosis
Box 45.1. Histological Classification of Hepatoblastoma
Epithelial |
Fetal Embryonal Small cell undifferentiated (bad prognostic subtype even when present focally) Macrotrabecular (bad prognosis but not a well-characterized subtype) |
Mixed epithelial–mesenchymal |
With teratoid features Without teratoid features |
Immunohistochemistry
♦
α-Fetoprotein positive (fetal and embryonal cells)
♦
βhCG positive (giant cells)
♦
Vimentin positive (anaplastic cells)
♦
Hepatocyte-specific antigen positive
♦
β-catenin positive
Staging
♦
There are two main staging systems for hepatoblastoma
♦
The Pretreatment Extent of Disease (PRETEXT) staging system is formulated by the International Childhood Liver Tumor Strategy Group (SIOPEL) and is used for staging tumors before the initiation of any therapy (Box 45.2)
♦
The Children’s Oncology Group (COG) staging system is formulated by the American Children’s Oncology Group. The COG system assesses completeness of resection, which is the single most important prognostic factor for hepatoblastoma (Box 45.3)
Box 45.2. PRETEXT Staging System for Hepatoblastoma
Stage I: one section involved and three adjoining sections free of tumor |
Stage II: one or two sections involved with two adjoining sections free of tumor |
Stage III: two or three sections involved and no two adjoining sections free of tumor |
Stage IV: All four sections involved |
Definition of liver sections used in PRETEXT staging: |
Left lateral section: segments 2 and 3 Left medial section: segments 4a and 4b Right anterior section: segments 5 and 8 Right posterior section: segments 6 and 7 |
Box 45.3. COG Staging System for Hepatoblastoma
Stage I: tumor completely resected with no microscopic residual disease |
Stage II: microscopic residual disease present or preoperative/intraoperative rupture |
Stage III: tumor unresectable or tumor resected with grossly visible residual disease or nodal involvement |
Stage IV: distant metastasis |
Liver Tumors with Biliary Differentiation
Benign
Biliary Hamartoma ( von Meyenburg Complex )
Clinical
♦
Biliary hamartoma is a common incidental finding at surgery or autopsy
♦
During surgery, it may appear as a metastatic deposit and is frequently biopsied for frozen section diagnosis
♦
There are often multiple lesions scattered randomly in the liver
♦
Biliary hamartoma is thought to represent a localized form of ductal plate malformation
Macroscopic
♦
Biliary hamartoma appears as small white nodule, a few millimeters in size
Microscopic
♦
Biliary hamartoma consists of irregularly shaped, dilated ducts in a fibrous stroma (Fig. 45.28)
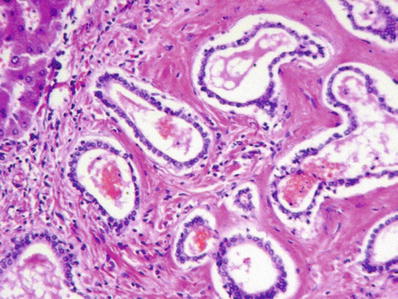

Fig. 45.28.
Biliary hamartoma consisting of dilated ducts containing eosinophilic material in a fibrotic stroma.
♦
Ducts may contain bile
♦
No cytologic atypia or mitotic activity
Differential Diagnosis
♦
Bile duct adenoma
Bile Duct Adenoma
Clinical
♦
Bile duct adenoma is an incidental finding present in up to 30% of individuals at surgery or autopsy
♦
Because it appears as a small white nodule studding the surface of the liver, bile duct adenoma may appear to be a metastatic deposit
It is frequently biopsied for frozen section diagnosis to rule out metastatic disease
Macroscopic
♦
Bile duct adenoma appears as a small, white, and firm subcapsular nodule
Microscopic
♦
Bile duct adenoma consists of small tubules set in a fibrous stroma (Fig. 45.29)
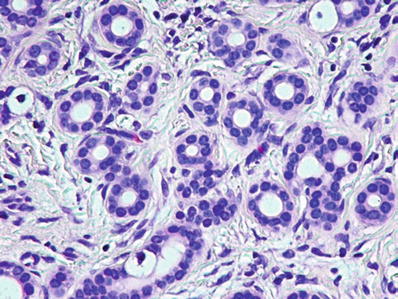

Fig. 45.29.
Bile duct adenoma consists of small benign tubules with regular nuclei and no mitoses in a fibrous stroma.
♦
The tubules are lined by a single layer of cuboidal cells
They may contain mucin, but bile is not present
This reflects their origin from peribiliary glands; “peribiliary gland hamartoma” is a more accurate term
♦
Entrapped portal tracts are often present within the lesion (Fig. 45.30)
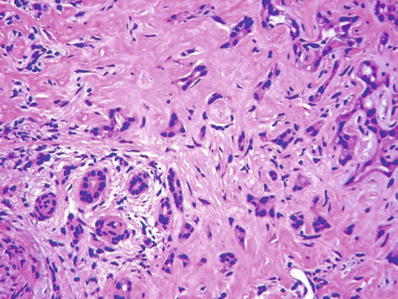

Fig. 45.30.
Bile duct adenoma with an entrapped portal tract within the lesion (left lower corner).
♦
Considerable nuclear atypia and desmoplasia may be present
These features should not be mistaken for metastatic carcinoma, especially in frozen sections (Figs. 45.31 and 45.32)
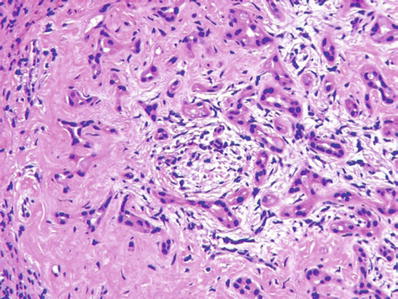
Fig. 45.31.
Bile duct adenoma showing considerable nuclear atypia and desmoplasia. The lesion was detected while the patient was undergoing Whipple excision for pancreatic carcinoma.
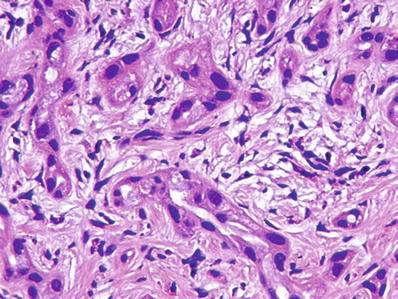
Fig. 45.32.
Bile duct adenoma showing considerable nuclear atypia and desmoplasia. High power of lesion shown in Fig. 45.31.
Differential Diagnosis
♦
Biliary hamartoma (von Meyenburg complexes)
♦
Mesenchymal hamartom a
Premalignant and Malignant
Mucinous Cystic Neoplasm
♦
This is also referred to as hepatobiliary cystadenoma
The term mucinous cystic neoplasm was proposed in the 2010 WHO classification of liver tumors
This term is more appropriate because mucinous cystic neoplasm of the liver is analogous to mucinous cystic neoplasm of the pancreas
Clinical
♦
Mucinous cystic neoplasm is a tumor of middle-aged women who present with pain and discomfort
The tumor grows slowly and tends to become symptomatic with increasing size
♦
There is a small risk of malignant transformation
♦




Surgical resection is curative
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


