Introduction
Sepsis is defined as symptoms and signs of systemic infection caused by a pathogen in the blood. Meningitis is defined as inflamed meninges typically caused by a pathogen in the central nervous system. Because neonates have an immature immune system, they are at increased risk for sepsis and meningitis caused by bacterial pathogens. Complement, granulocyte, humoral, macrophage, and T cell functions are decreased in neonates (compared to older pediatric and adult populations), limiting response to pathogens. Skin, a protective barrier to pathogen exposure, is often immature and compromised when obtaining laboratory tests and performing procedures.1 Neonatal infections are classified by time as either early onset or late onset. Early-onset infections or early-onset sepsis (EOS) occur during the first 72 hours of life, and late-onset infections or late-onset sepsis (LOS) occur after 72 hours of life.
Early-Onset Infections
Presentation and Laboratory Evaluation
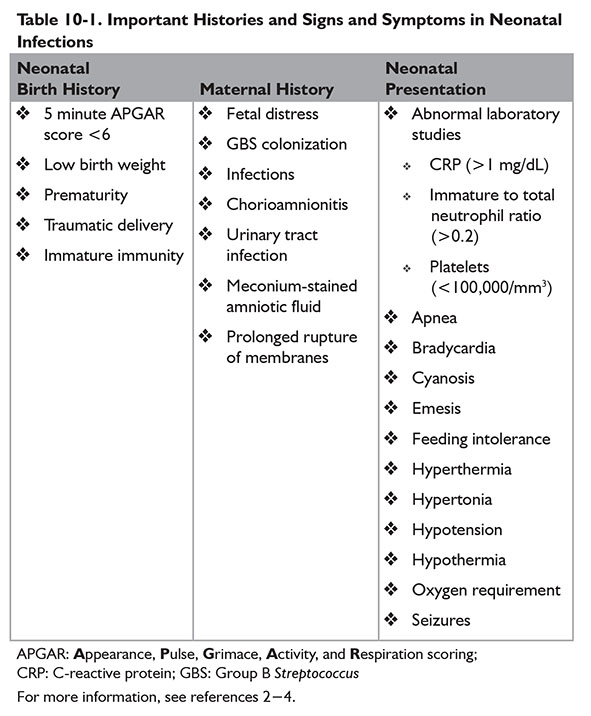
Signs and symptoms of early-onset infections often mirror signs and symptoms of other neonatal conditions such as respiratory distress or apnea of prematurity. Neonatal birth history and maternal obstetric history may increase risk of early-onset infections. Key histories and neonatal signs and symptoms for suspected early-onset infections are provided in Table 10-1. If indicated, cultures should be obtained from blood and cerebral spinal fluid (CSF). Urine cultures have minimal diagnostic value in early-onset infections.
Complete blood cell counts with differential may be used to aid diagnosis of early-onset infections in neonates. Sensitivities to rule out EOS in neonates are low when using complete blood cell count indices. However, patients with a low white blood cell (WBC) count, low absolute neutrophil count (ANC), or high immature to total neutrophil percentage (i:T ratio) have an increased probability of being infected.5 Escobar and colleagues also noted increased odds of sepsis in neonates weighing ≥2 kg at birth with a low ANC.6 Abnormal WBC counts, bands, segmented neutrophils, and platelets used jointly assist in identifying infected neonates.7
Elevated C-reactive protein (CRP) may also indicate presence of infection.8 CRP increases within 8 hours of infection and peaks around 24 hours. Values obtained within the first few hours after birth should not be used in assessment. Two normal CRP values obtained within the first 48 hours of life approximately 12 to 24 hours apart is a negative predictor of infection.9,10 Elevated procalcitonin (PCT) concentrations may also be used to identify infection in neonates. PCT increases within 2 to 4 hours after infection and peaks around 6 to 12 hours, potentially allowing for earlier identification of infection. However, neonates have a physiologic increase in PCT during the first day of life. Using PCT alone is not recommended at this time.9,11
A group of abnormal biomarkers is more indicative of infection in neonates than a single abnormal biomarker.7,8 Other biomarkers studied or currently being studied include interleukins 1β, 6, and 8 as well as tumor necrosis factor α and neutrophil cluster of differentiation 11β and 64.8,10,11
Common Pathogens
In neonatal populations, the two most common pathogens in early-onset infections are Streptococcus agalactiae (group B Streptococcus [GBS]) and Escherichia coli (E. coli) in the era of maternal intrapartum antibiotic prophylaxis (IAP) for GBS.2,12,13 The most common pathogen in term neonates is GBS compared to E. coli in preterm neonates.2 The incidence of early-onset GBS infections decreased from 3.5 cases per 1,000 NICU admissions 5 years prior to introduction of universal IAP to 2.6 cases per 1,000 NICU admissions the following 9 years. Early-onset E. coli infections remain consistent at 1.4 cases per 1,000 NICU admissions during the 14-year analysis.14
Pharmacologic Treatment
To limit neonatal morbidity and mortality, empiric antibiotic treatment should be initiated when early-onset risk factors are present or infection is suspected.1,15 The selected regimen should be efficacious against common pathogens. Neonates are often started on ampicillin and gentamicin for early-onset infections.2,9,12 Third-generation cephalosporins such as cefotaxime may be initiated instead of an aminoglycoside in combination with ampicillin. However, bacterial resistance to cefotaxime may develop quicker compared to aminoglycosides.16
There may also be an increased risk of invasive fungal infections when third-generation cephalosporins are used to treat early-onset infections.17,18 Cefotaxime should be reserved for patients with meningitis.9 Empiric antibiotic dosing regimens of ampicillin and aminoglycosides vary based on age (postconceptual, postnatal, and/or gestational age) and/or weight, depending on institution-preferred neonatal and pediatric dosing reference.19,20 When using aminoglycosides, therapeutic drug monitoring should be performed by obtaining serum peak and trough concentrations or obtaining serum concentrations for patient-specific pharmacokinetic determinations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree