Chapter 28 Antiprotozoal natural products
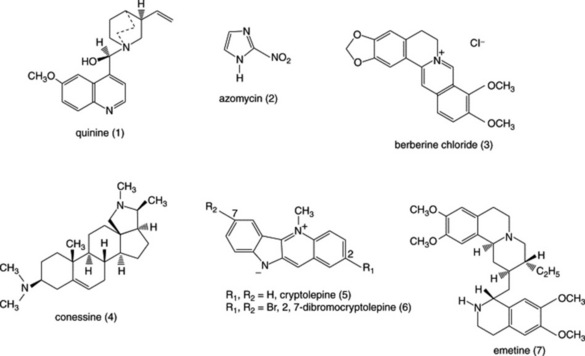
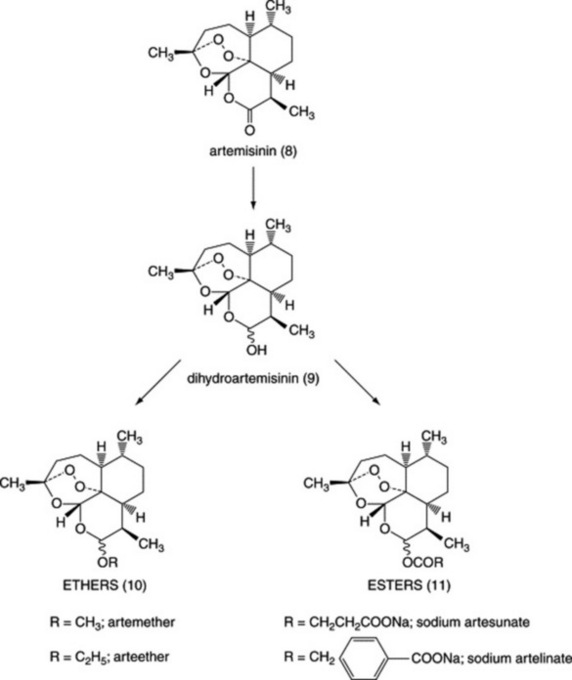
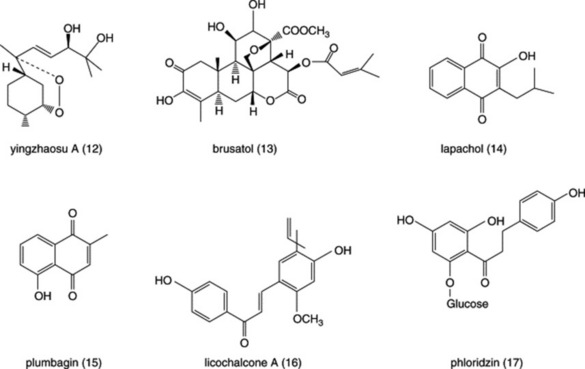
Diseases caused by protozoa are responsible for considerable mortality and morbidity, especially in the developing world. Many plant species are used in the preparation of traditional medicines for the treatment of protozoal diseases and plants are the source of the clinically used antimalarial drugs quinine (Fig. 28.1 (1)) (from Cinchona spp.) and artemisinin (Fig. 28.3 (8)) (from Artemisia annua). Other examples of natural-product-derived antiprotozoal agents are the nitroimidazoles, which are based on the antibiotic azomycin (Fig. 28.1 (2)) produced by a species of Streptomyces that was collected on the Island of Réunion. Azomycin was found to be active against the protozoan Trichomonas vaginalis, the causative agent of trichomoniasis, and the synthetic analogue metronidazole was the first effective treatment for this disease. Later, it was found that the latter was also highly effective in the treatment of infections caused by anaerobic bacteria. A more recent example of a natural-product-derived antiprotozoal drug is the antimalarial atovaquone, which was derived from lapachol (Fig. 28.4 (14)), a naphthoquinone found in S. American species of the Bignoniaceae. Natural products have made a significant contribution to the chemotherapy of protozoal diseases and it is possible that the continued investigation of natural-product-derived compounds will provide new antiprotozoal drugs in the future. As shown in the following sections, many of the available antiprotozoal agents have serious limitations due to their toxicity and/or the development of drug-resistant parasites, so that new drugs are urgently needed.
DISEASES CAUSED BY PROTOZOA
Trypanosomiasis
African sleeping sickness (African trypanosomiasis) is caused either by Trypanosoma brucei gambiense (W. African form) or by T. brucei rhodesiense (E. African form). The disease is transmitted by the tsetse flies (Glossina species) and initially causes a feverish illness. Later stages of the disease are characterized by effects on the central nervous system, including movement disorders and convulsions, excessive sleepiness and finally coma. About 50 million people live in areas where the disease is endemic and, in addition to the threat to humans, it is also a majorproblem for livestock. Current drug treatment is limited as suramin and pentamidine are only effective in the early stages of the disease while the arsenical drug melarsoprol, which is used in the late stages, is toxic. Treatment has improved with the development of eflornithine (α-difluoromethylornithine), an inhibitor of polyamine synthesis but this drug is not effective against the E. African form of the disease.
METHODS OF INVESTIGATION
Antimalarial assays
In vitro (antiplasmodial) assays
Plasmodium falciparum is cultured in human red blood cells in 96-well microtitre plates. The inhibition of parasite growth in the presence of drugs may be assessed by measuring the incorporation of [3H]-hypoxanthine into the parasite. More recently, a new method has been developed that does not require the use of radiolabelled compounds. Instead, parasite growth is assessed by measuring parasite lactate dehydrogenase (LDH) activity by adding a reagent containing an analogue of NAD, acetylpyridine adenine dinucleotide (APAD) and a tetrazolium compound. APAD is reduced by parasite LDH (but not by red cell LDH) and then the reduced APAD in turn reduces tetrazolium to give a blue colour, which is measured spectrophotometrically; the intensity of the colour is proportional to parasite growth.
< div class='tao-gold-member'>