and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
4.1 Definition, Site and Incidence
Myoepithelioma (ME) is defined as a tumour composed of myoepithelial cells that exhibit spindle, epithelioid, plasmacytoid or clear cytoplasmic features. The tumour is composed of myoepithelial cells alone or with the addition of small numbers of ductal structures. The cells are arranged in different patterns and grow in a mucoid, collagenous or vascular stroma. Myoepitheliomas were previously thought to lack the myxoid or chondroid stroma typical of pleomorphic adenoma, but such stromal changes are nowadays recognised to be present. Myoepithelioma may hence histologically be very similar to pleomorphic adenoma, and the scarcity of duct-like structures may be the only distinguishing feature. Their significantly lower rate of recurrences and malignant change than that of pleomorphic adenomas merit their distinction as a separate entity. Myoepitheliomas have the histology and growth patterns of the myoepitheliomatous component of pleomorphic adenomas, but hence lack or have only limited (<5–10 %) differentiation of the ductal phase of this tumour [10, 11]. They are encapsulated or at least well-circumscribed tumours and the majority arise in the parotid gland. The proportion of parotid myoepitheliomas range from 40 to 90 % in different series and of intraoral minor salivary glands; the palatal glands are the most commonly affected. ME rarely arises in the submandibular gland and it appears as only approximately ten cases have been reported in the English literature [48]. A few cases of ME have been described in the sinonasal tract [19, 30, 40], larynx [9] and trachea [8]. Myoepitheliomas account for 1–1.5 % of all salivary gland neoplasms and represent 2.2 % of all benign major salivary gland tumours and 5.7 % of benign minor salivary gland tumours [1, 7, 11, 14, 44]. Myoepitheliomas are well known to arise in the skin and soft tissues [25, 38]. MEs have also been reported from the breast [33, 42, 47] and bone [29, 31, 35]. Only half a dozen cases of primary lung myoepithelioma have been reported [34] but also a myoepithelioma arising in a classic lung hamartoma [41]. The first case of an ovarian ME was recently described [26]. A case of metachronous bilateral myoepithelioma of the nasopharynx has been reported [20] as well as a ME of the buccal mucosa metachronous with an upper lip polymorphous low-grade adenocarcinoma [4]. Myoepitheliomas are very rare in children and only a few cases are reported [23, 51].
4.2 Clinical Features and Gross Appearances
There are no distinctive clinical features but myoepitheliomas usually present as slowly growing, asymptomatic masses. The age and sex distribution are similar to that of pleomorphic adenoma, i.e. a slight female predilection and most common in adults between the ages of 30 and 50. They are rather solid tumours and are usually small measuring less than 3 cm and hence rarely reach the large size of many pleomorphic adenomas. The cut surface varies but is often tan or yellow-tan but seldom so glistening as can be the case in pleomorphic adenomas. Parotid pleomorphic adenomas often tend to bulge out from the parotid tissue only being surrounded by a thin capsule; however, this feature is rarely seen in myoepitheliomas.
4.3 Histopathology
Myoepitheliomas are encapsulated tumours and may consist of any combination of the main four different morphological cell types, but in the majority of cases, there is one cell type that predominates and only in smaller areas one will see one or two other types. It is a tumour entity with heterogeneous cytomorphology and inconsistent immunophenotype. There may be different growth patterns such as solid (non-myxoid), myxoid (pleomorphic adenoma-like) and reticular. Regardless of the architectural pattern, the uniformity of the tumour is a striking feature. In this respect, they can more easily be confused with basal cell adenoma than pleomorphic adenoma. A solid growth pattern is the most common where the cells are organised in nests and sheets separated by limited or variable amounts of fibrous or hyalinised stroma. The stroma may be well vascularised, and there is no, or very scarce, amount of myxoid background. In myoepitheliomas with a myxoid or pleomorphic adenoma-like growth pattern, there are variable amount of myxoid foci. Here the tumour cells either have a mesh-like or sieve-like architecture. They may also be scattered as small, irregularly sized and shaped groups of cells within the mucinous background. On very rare occasion, the growth pattern can be reticular or canalicular-like.
In spite of the above-mentioned variations, the histological appearance of myoepithelioma is still somewhat monotonous with a tumour consisting of packed, often rather small cells with scanty cytoplasm. The nuclei are regular and oval in shape and the cell membranes are ill defined (Fig. 4.1). The cells can be more epithelioid and in these cases the stroma tends to be more abundant (Fig. 4.2). These types of myoepitheliomas stain positively for p63, cytokeratin and S-100, whilst smooth muscle actin is weakly expressed or in fact frequently absent (Fig. 4.3). The typical myxoid or chondroid stroma seen in pleomorphic adenoma is occasionally present in myoepithelioma and does thus not exclude the diagnosis of myoepithelioma. The stroma in myoepitheliomas is frequently hyalinised as in pleomorphic adenomas, but there are only few ductal structures present, if any. The myoepithelial cells can appear as being embedded in the hyalinised stroma or appear as solid tumour nests separated by the hyalinised stroma (Fig. 4.4). Many myoepitheliomas are characterised by solid masses of spindle-shaped myoepithelial cells arranged in whirling bundles. Contrary to cases of myoepitheliomas consisting primarily of cells expressing epithelioid, plasmacytoid or clear cytoplasmic features, the spindle-shaped cells tend to express myogenic markers (Fig. 4.5). Parts of myoepitheliomas may show cells arranged in an ‘Indian file’ pattern, and yet in other areas, the myoepithelial cells can be arranged in a canalicular pattern (Fig. 4.6). On rare occasions, the tumours may be hypocellular in an oedematous stroma and the cells almost stellate. The features can be very similar to a pleomorphic adenoma, except that there are almost no ducts and no chondroid stroma (Fig. 4.7). Many blocks have to be taken in these cases to exclude pleomorphic adenoma. Cystic myoepitheliomas are not common but not by any means rare. Contrary to its malignant counterpart, myoepithelial carcinoma, which often has microcysts, myoepitheliomas tend to have a few smaller macrocysts (Fig. 4.8). There are also frequent ‘punched out’ holes as seen in many other salivary gland tumours. Also areas with scattered lipometaplasia may appear like small cysts or ‘punched out’ holes. Although the majority of the macrocysts are small, they may occasionally be large enough to be detected by sonography [22]. Some of the cysts are filled with an eosinophilic fluid, some are not. The cysts have no special epithelial lining but it is still reasonable to believe that they represent remnants of dilated ducts. There is no accompanying lymphoid stroma as frequently seen in Warthin tumour and acinic cell carcinoma. Calcium deposits are occasionally found but less common in comparison with pleomorphic adenoma, where the calcium often is found adjacent to the cysts. The calcium deposits tend to be located in the ‘punched out’ cystic structures (Fig. 4.9). Oncocytic and lipomatous metaplasia occur in myoepithelioma, however, not as frequently as in pleomorphic adenomas [32, 45]. Tyrosine-rich crystalloids, which are thought to be almost exclusively found in pleomorphic adenoma, have been described in a case of palatal myoepithelioma [24].
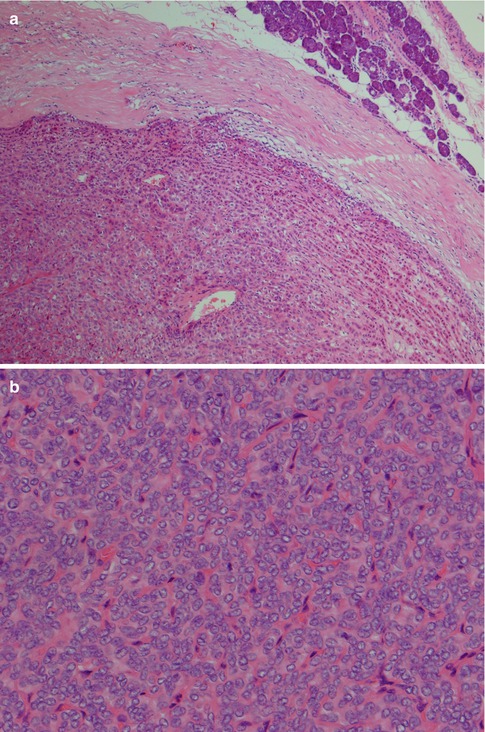
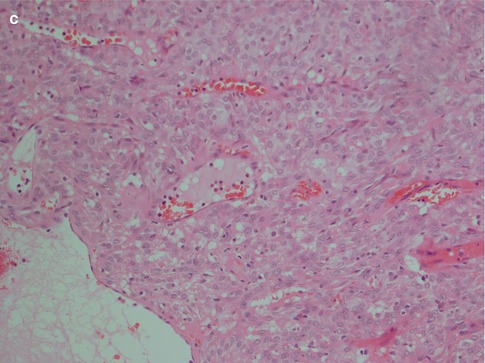
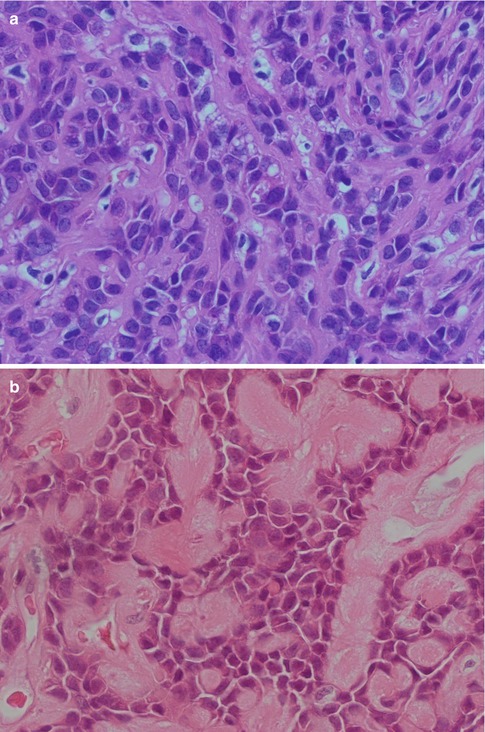
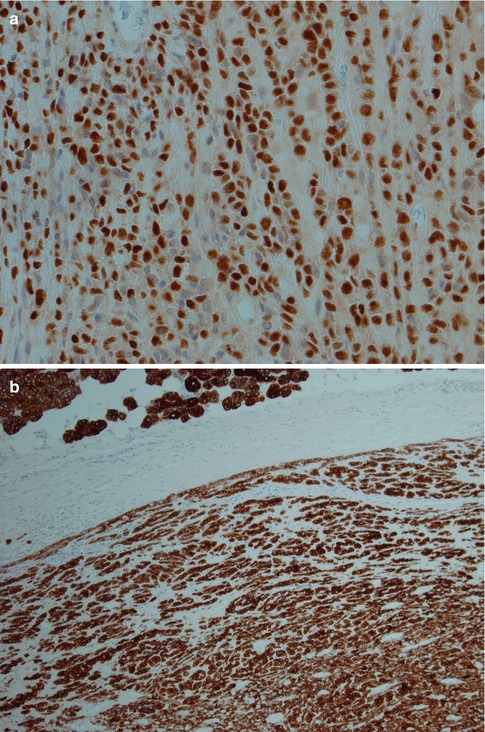
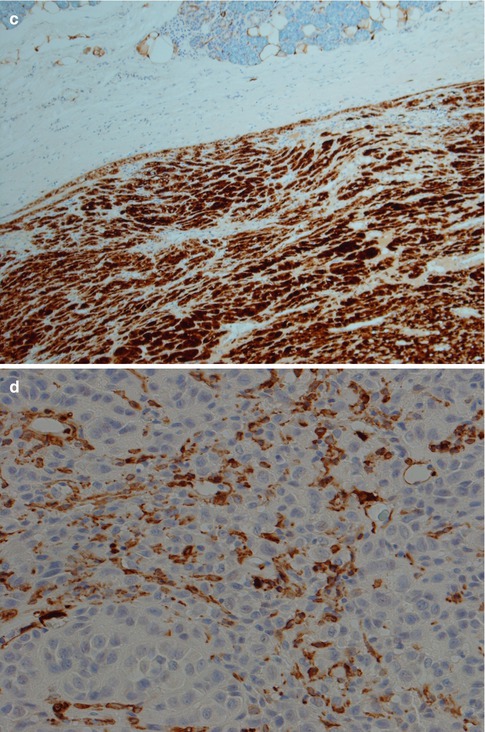
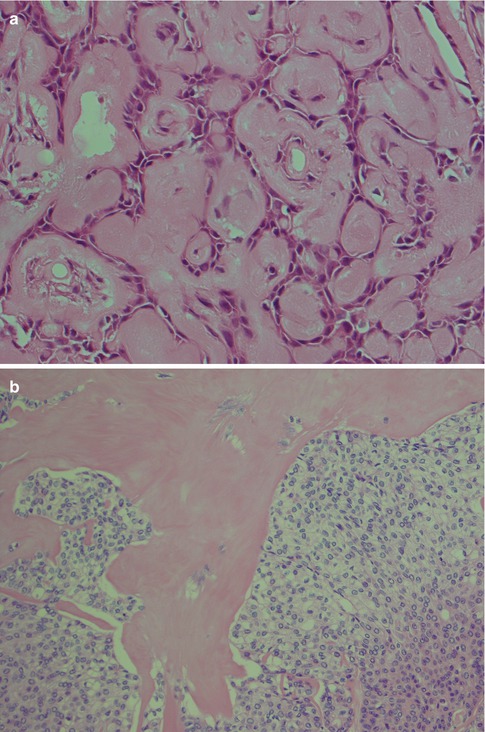
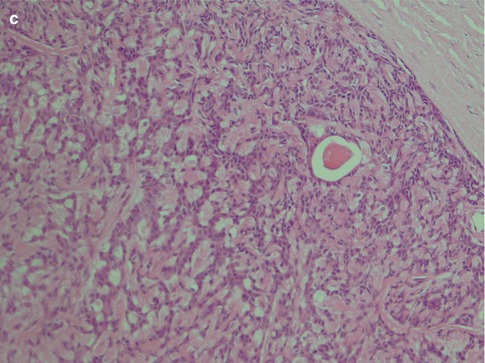
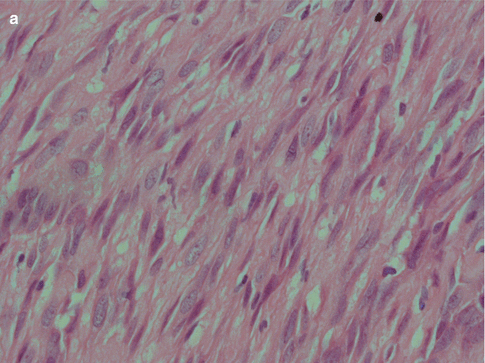
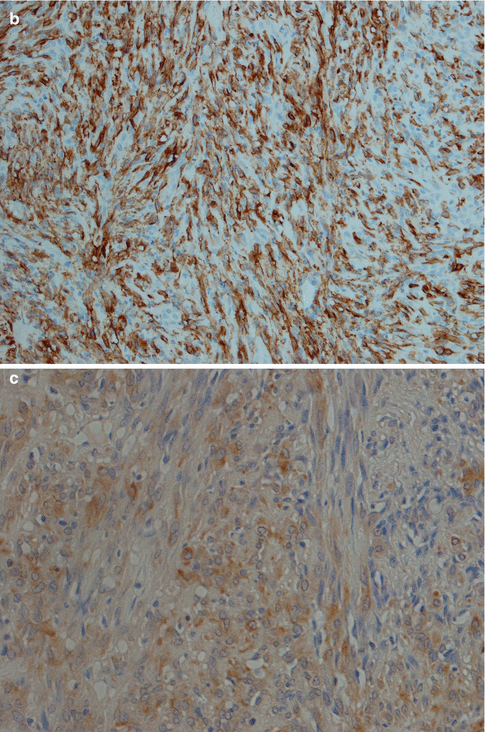
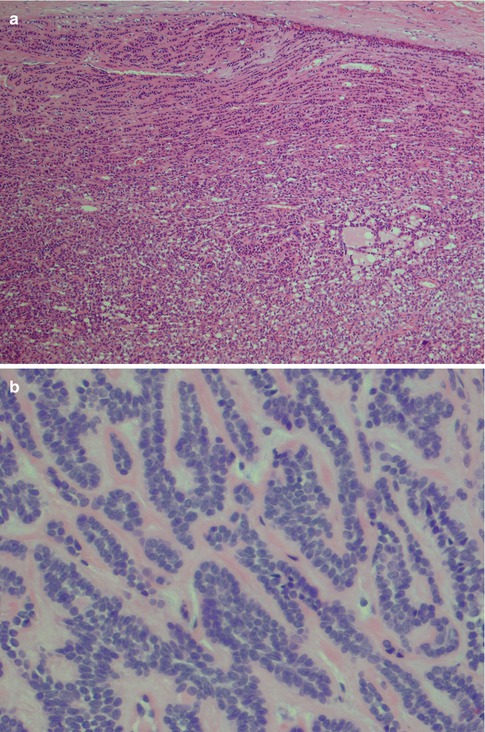
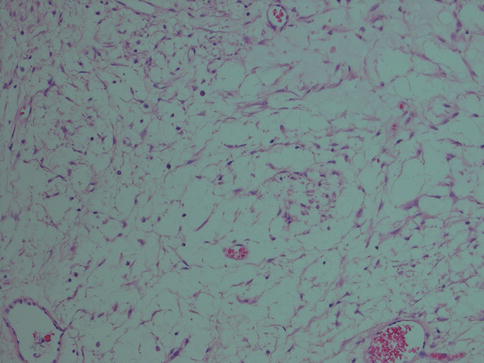
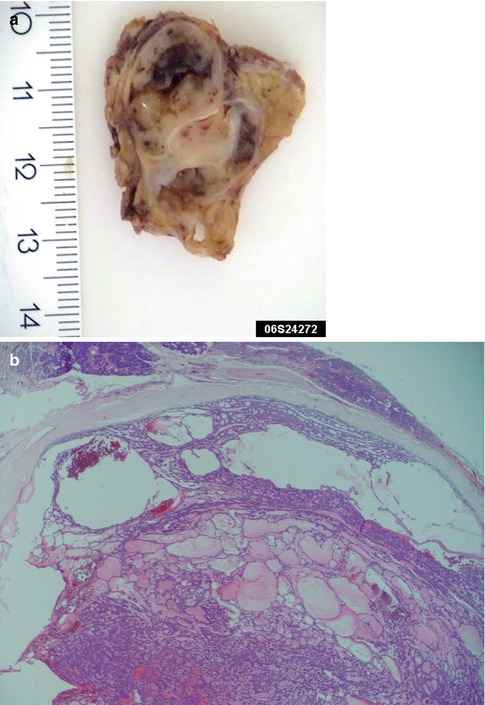
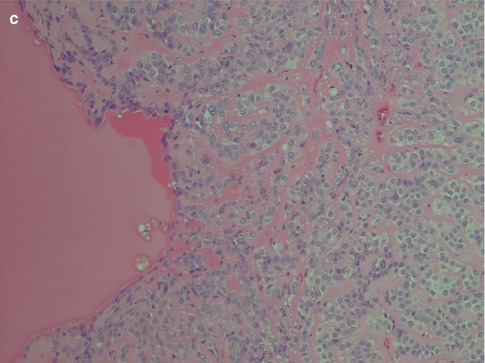
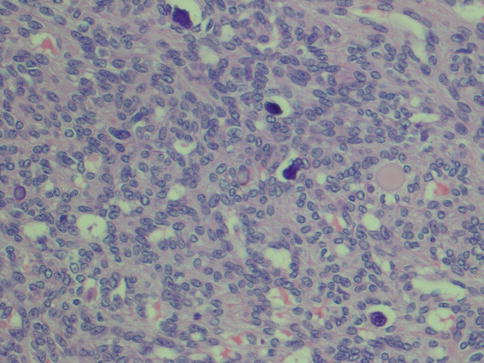


Fig. 4.1
(a) Cellular parotid myoepithelioma with a thick fibrous capsule. (b) The tumour consists of densely packed small oval cells with vesicular nuclei and scanty cytoplasm. (c) Richness of vessels in between the closely packed myoepithelial cells

Fig. 4.2
(a) Myoepithelioma with predominantly epithelioid cells and scattered clear cells. (b) Predominantly epithelioid myoepithelioma with plasmacytoid features. More abundant and also hyalinised stroma




Fig. 4.4
(a) Myoepithelioma with a hyalinised stroma. (b) Another ME where nests of myoepithelial cells are separated by a hyalinised stroma. (c) Myoepithelioma with spindle-shaped cells and the odd ductal structure


Fig. 4.5
(a) ME consisting primarily of spindle-shaped cells. (b) Spindle cell myoepithelioma staining positive for SMA (compare to Fig. 4.3d). (c) The same tumour showed focal positivity for GFAP

Fig. 4.6
(a) Myoepithelioma with spindle-shaped and clear cells, focally arranged in ‘Indian file’ growth pattern (top). (b) Another ME where the cells are arranged in a canalicular fashion with two layers of myoepithelial cells (canalicular adenoma consists of ductal p63 negative cells. See Chap. 6)

Fig. 4.7
Myoepithelioma with mesh-like or sieve-like architecture with stellate cells in an oedematous stroma


Fig. 4.8
(a) Gross appearance of a multicystic myoepithelioma. (b) Microscopy of the same tumour. (c) Another case of ME with macrocysts

Fig. 4.9
Cellular palatal myoepithelioma with calcium deposits
4.3.1 Immunohistochemistry
The immunophenotype is similar to pleomorphic adenoma. The characteristic co-expression of CK14, actin, p63 and S-100 seen in normal myoepithelial cells (see Chap. 1) may or may not be present in neoplastic myoepithelial cells. Particularly the myogenic-type markers tend to be negative in many myoepitheliomas, whilst S-100, cytokeratin, p63 and vimentin are more commonly expressed. Particularly plasmacytoid types of myoepithelioma are negative for both smooth muscle actin, muscle specific actin and CK14 [17, 43]. Low molecular weight keratins such as CK8/18 are supposed to be negative in normal myoepithelial cells but are positive in many myoepitheliomas. CK8/18 (and pancytokeratin) stain the few ductal structures present more intensively than the myoepithelial cells, and these immunostains may therefore be used as an aid in the differential diagnosis between myoepithelioma and pleomorphic adenoma, the latter with an abundance of ducts (see Chap. 3). Recently a study showed that tumour cells with myoepithelial differentiation, such as ME and PA, are immunopositive for podoplanin (D2-40). Podoplanin is known as a representative marker for lymphatic endothelium, and the authors suggest that podoplanin is a novel myoepithelial marker in salivary gland tumours [49].
Gnepp recently drew attention to the existence of a variant of ME, the so-called mucinous myoepithelioma that possibly merits to be classified as a separate entity [21]. There are apparently 17 reported cases that would fit into this category; however, only 4 of the 17 cases were benign. Its biological behaviour is unclear but the tumour appears to be benign to low-grade malignancy. Some of the cases in the literature have been reported as signet ring-cell adenocarcinoma. The tumours stained for myoepithelial markers and contained intracellular mucin [21]. The fact that 13 of the 17 tumours were classified as malignant may warrant some caution before designating it as mucinous myoepithelioma, a term that indicates a benign neoplasm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


