Myoepithelial Neoplasms
EDI BROGI
THE MYOEPITHELIAL CELL
Myoepithelial cells (MECs) comprise part of the normal lining of mammary ducts and lobules.1 They are interposed between the polarized glandular cells and the underlying basement membrane in the so-called basal cell layer. These cells have contractile properties similar to smooth muscle cells and express smooth muscle-specific proteins such as smooth muscle actin (SMA). Studies of mammary and salivary gland tissue have shown that MECs derive from the ectoderm, whereas smooth muscle cells derive from the mesoderm.2,3 Differentiation of epithelial cells and MECs has been observed in the terminal end buds of the developing pubertal breast.4 O’Hare et al.5 used fluorescence-activated cell sorting to separate epithelial membrane antigen (EMA)-positive luminal cells and CD10-positive MECs from lysates of fresh mammary tissue and studied the cytologic and immunophenotypic characteristics of these two cell types. Additional and more sophisticated methods for purification and study of MECs have also been developed, as reviewed by Clarke et al.6
During lactation, mammary MECs contract upon stimulation by oxytocin, resulting in the expression of milk from the nipple ducts. Recent observations7,8 have demonstrated “tumor-suppressor” properties of MECs that are mediated through physical and paracrine activity. MECs form a barrier between the ductal epithelium composed of luminal cells and the surrounding stroma, thereby preventing direct interaction between the two tissue components. This function is especially important when the glandular epithelium consists of carcinoma in situ (CIS). In addition, MECs produce components of the basement membrane, as well as antiangiogenic and antiprotease factors, contributing to maintain a controlled microenvironment that wards off stromal invasion. It has been hypothesized that local chemokines and/or inflammation can result in physical and functional attenuation of the myoepithelial layer, weakening the basement membrane and creating local conditions that are permissive of stromal invasion.8 On the other end of the spectrum, some investigators hypothesize that MECs, or a population of basal stem cells admixed with MECs, are involved in the development of basal-like mammary carcinomas. However, using a murine model of mammary carcinoma, Molyneux et al.9 found that deletion of the BRCA1 gene in the basal cells/MECs led to the development of malignant adenomyoepitheliomas (AMEs). In contrast, deletion of the same gene in the luminal epithelium produced tumors with features of basal-like carcinomas, challenging the hypothesis that basal-like carcinomas derive from basal stem cells presumed to reside in the myoepithelial layer.
This chapter is devoted to a discussion of neoplasms that display the distinctive phenotype of MECs. Carcinomas referred to as “basaloid” or “basal-type” are considered elsewhere in this volume, including in Chapter 12.
MEC Morphology
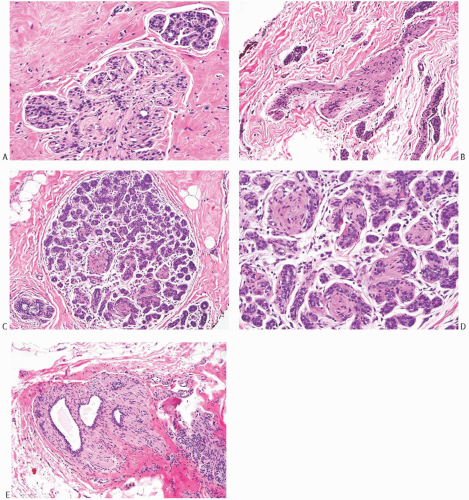
MECs can have spindle or epithelioid morphology. They are usually inconspicuous, unless they are hyperplastic. In histologic sections, the nucleus of spindle-shaped MECs is located in the center of the cell. It is elongated, with the longest axis parallel to the basement membrane. Although minimally visible in routine hematoxylin and eosin (H&E)-stained sections, the cytoplasm of MECs is readily apparent in sections immunostained for myofilaments such as calponin or SMA. Mammary MECs sometimes acquire polygonal or globoid morphology, with abundant clear cytoplasm and pseudovacuoles. This appearance not only constitutes a physiologic alteration during the luteal phase of the menstrual cycle,10 but is also commonly found in sclerosing lesions (Fig. 6.1) and in myoepithelial tumors. MECs with clear cytoplasm can mimic atypical lobular hyperplasia (ALH) and classic lobular carcinoma in situ (LCIS) with pagetoid growth, but the uniform circumferential distribution at the periphery of the acini is usually sufficient for correct identification. Immunoperoxidase stains for MEC markers can help resolve problematic cases (see also Chapter 31 for a more detailed discussion of this differential diagnosis). Clear cell change and subtle hyperplasia of the MECs are also common in irradiated breast (Fig. 6.1). The pseudovacuoles of MECs lack mucin and do not stain with Alcian blue and periodic acid-Schiff (PAS) stains. Myoepithelial hyperplasia sometimes can nearly obliterate the acinar lumen, resulting in an appearance that mimics classical LCIS or ALH. In these cases, an E-cadherin stain will show expansion by an MEC population with discontinuous and granular membranous reactivity of weaker intensity than in the ductal cells. This pattern of reactivity should not be interpreted as evidence of lobular differentiation, and immunostains for calponin and p63 will complement the diagnosis (Fig. 6.2).
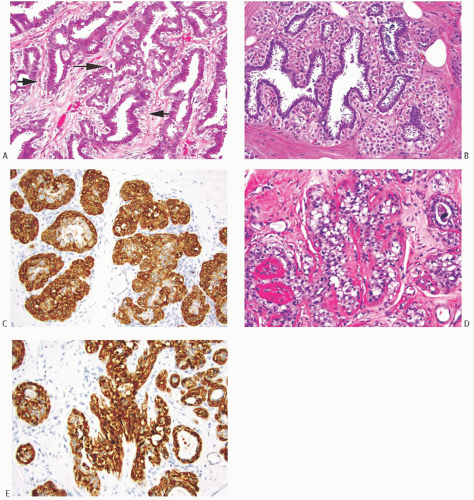 FIG. 6.1. Myoepithelial cells, epithelioid morphology. A: Epithelioid MECs in a papilloma have globoid shape and abundant clear cytoplasm (short arrows). The nuclei are slightly enlarged and ovoid, with visible nucleoli. A mitotic figure is evident (long arrow). B: Hyperplastic epithelioid MECs in a lobule. This pattern may be mistaken as pagetoid spread of ALH or classical LCIS. C: The MECs in (B) are reactive for calponin. D: The MECs in this irradiated lobule are slightly hyperplastic and have relatively abundant clear and vacuolated cytoplasm. Almost complete absence of epithelial cells and the thickened basement membranes are consistent with radiation effect. E: An immunoperoxidase stain for calponin in the same tissue as in (D) highlights the hyperplastic myoepithelium. |
Myoid transformation of MECs is commonly observed as an incidental finding in breast tissue from premenopausal and postmenopausal women. When this occurs, the MECs acquire the cytologic and histochemical features of smooth muscle cells, including a more pronounced spindle shape and eosinophilic cytoplasm. Myoid transformation is most frequently encountered around terminal ducts and lobules in the absence of appreciable epithelial proliferation (see Chapter 1) (Fig. 6.3). These changes are not associated with any particular type of tumor and may be found in specimens from patients who had breast tissue sampled for various benign or malignant lesions. Myoid transformation is often present in the foci of sclerosing adenosis, and it may occasionally dominate the process, leading to a leiomyomatous appearance (Fig. 6.4).
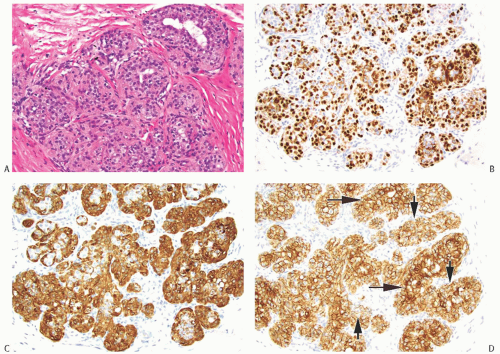 FIG. 6.2. Myoepithelial hyperplasia that mimics classical LCIS. A: In this example of florid myoepithelial hyperplasia, most of the involved acini have no visible lumens, and the overall appearance mimics classic LCIS or ALH. B: A p63 immunostain highlights the nuclei of the hyperplastic MECs. C: A calponin immunostain decorates the cytoplasm of the hyperplastic MECs. D: Membranous immunoreactivity for E-cadherin in the hyperplastic myoepithelium (short arrows) is less intense than that in the luminal cells (long arrows) lining the acini. Attenuated staining reflects the normal staining pattern of MECs, and it should not be interpreted as suggestive of lobular neoplasia. |
Immunohistochemistry
In addition to SMA, the cytoplasm of MECs is also immunoreactive for smooth muscle myosin heavy chain (SMM-HC), calponin, and CD10.11 S-100 and maspin decorate the cytoplasm and the nucleus of MECs, whereas p75 highlights the cytoplasm and the cell membrane.11 MECs are characterized by nuclear immunoreactivity for p63, a p53 homolog protein. Glial fibrillary acidic protein (GFAP) and caveolin-1 are myoepithelial antigens less frequently used for the identification of mammary MECs. Epithelial markers such as CK5, 6, 14, and 17, keratin 34βE12, and P-cadherin also stain the mammary MECs. E-cadherin decorates the myoepithelium with a characteristic membranous “dot-like” discontinuous linear pattern. Keratin AE1 does not stain the mammary myoepithelium, whereas AE3 stains the myoepithelium of the mammary ducts but not of the acini.12 MECs are usually negative for EMA, carcinoembryonic antigen (CEA), and CK8/18.
MECs in Breast Lesions
MECs participate in many benign proliferative processes in the breast, most notably sclerosing adenosis (see Chapter 7) and papillary proliferative lesions of ducts (see Chapter 5). Myoepithelial hyperplasia sometimes accompanies classical LCIS and ALH (see Chapter 31) and is also common in normal and hyperplastic mammary ducts and glands in the irradiated breast. It can also occur focally with no apparent reason (Figs. 6.2, 6.3 to 6.4). In contrast, MECs are often reduced around ducts involved by ductal carcinoma in situ (DCIS) with or without associated invasive carcinoma.
Mammary neoplasms composed partly or entirely of MECs are uncommon.13 The classification of these tumors is complicated by the inherent phenotypic plasticity of MECs. The MECs in the salivary gland epithelium contribute to the histogenesis of pleomorphic adenomas (mixed tumors) and carcinomas that arise in these glands.14,15 Morphologic similarities between certain tumors of the breast, salivary glands, and skin appendage glands reflect the contribution of MECs to these lesions.
A mammary neoplasm that exhibits epithelial and myoepithelial differentiation is referred to as AME. Most AMEs are benign tumors. When either the epithelial or the myoepithelial component of an adenomyoepitheliomatous tumor is malignant, the appropriate diagnosis is adenocarcinoma arising in an AME or myoepithelial carcinoma, depending on the nature of the malignant component. The term malignant AME should be reserved for exceedingly rare neoplasms in which both the epithelial and myoepithelial components
are malignant. Unfortunately, these distinctions have not been made in most of the published reports of adenomyoepithelial neoplasms. Classification is further complicated by uncommon tumors that exhibit combined adenomyoepithelial and microglandular adenosis-like (MGA-like) growth patterns.16,17
are malignant. Unfortunately, these distinctions have not been made in most of the published reports of adenomyoepithelial neoplasms. Classification is further complicated by uncommon tumors that exhibit combined adenomyoepithelial and microglandular adenosis-like (MGA-like) growth patterns.16,17
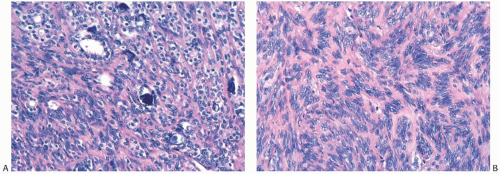 FIG. 6.4. Myoid differentiation in sclerosing adenosis. A: Myoid MECs surround glands with calcifications. B: Palisading of spindly MECs in nodular sclerosing adenosis. |
Benign neoplasms composed entirely of MECs are referred to as myoepitheliomas. Malignant MEC neoplasms with myoepithelial differentiation have been classified as myoepithelial carcinomas. This term applies to some neoplasms, especially those that qualify as myoepithelial carcinoma arising in an AME. However, the observation that many of the malignant neoplasms classified as metaplastic carcinomas express myoepithelial markers has clouded the distinction between myoepithelial carcinoma and metaplastic carcinoma from a histogenetic standpoint, although these entities are usually histologically distinguishable. Additional discussion of this topic in the context of metaplastic carcinoma can be found in Chapter 16.
ADENOMYOEPITHELIOMA
The first full description of AME of the breast was published in 1970 by Hamperl.18 With the exception of five studies consisting, respectively, of 6, 13, 18, 27, and 23 cases,19,20,21,22,23 most reports of AME have been case studies.
Clinical Presentation
Age, Gender, and Genetic Predisposition
Most patients with AME are women of postmenopausal age, but patients as young as 2620 and 2721 years have been reported. Two examples of AME have been described in men of age 4724 and 84 years.25 No malignant AME has been reported in male patients.
Presentation
Most patients present with a solitary, unilateral painless mass. Han and Peng27 reported one patient with three distinct AMEs, one of which was benign, one atypical, and the third contained DCIS. Most lesions occur in the periphery of the breast, but occasionally they have been found centrally or near the areola,22,28,29,30,31,32,33,34 including AMEs in two male patients.24,25 Nipple discharge,32 pain, and tenderness are infrequent. In some cases, the tumor was palpable for nearly a year before excision.21,22,35 Patients with malignant tumors may describe recent onset or report rapid growth of a longstanding lesion.
Imaging Studies
Mammography typically reveals a single, circumscribed mass that is not easily distinguished from a fibroadenoma. The border of the tumor is usually well circumscribed and sometimes microlobulated. An irregular contour is uncommon.30,31 In a few instances, the mammographic appearance has been interpreted as suspicious.30,31,36,37 Radiologically apparent calcifications are exceedingly rare.27,30,38,39 The presence of pleomorphic calcifications in a benign AME was considered to be a “suspicious” radiologic finding.40 AMEs examined by mammography usually present as palpable tumors measuring 3 cm or less in diameter,30 but there have been reports of small nonpalpable AMEs that were detected on mammography alone.36 Conversely, some palpable AMEs were not apparent in mammograms because they were obscured by superimposed dense breast tissue.31,38 Since AMEs resemble fibroadenomas mammographically, the lesions may sometimes be left in place and followed with imaging studies.21
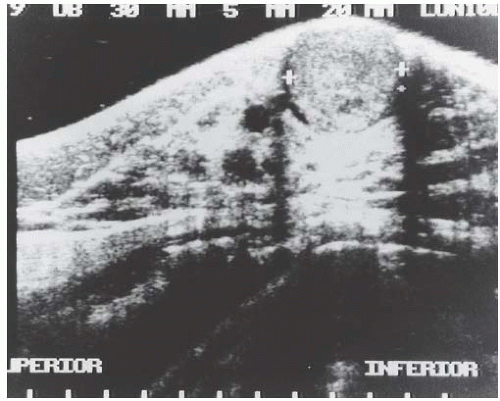
Sonographically31,38 AME appears as a solid, round or oval mass (Fig. 6.5) with hypo- or complex echogenic texture. The margin is typically smooth or lobulated, and less often irregular.31,36 Posterior acoustic enhancement may be present,36,37 depending on the cellular composition of the tumor (Fig. 6.5). Hypervascularity has been reported in the vicinity of AME,41 and ectatic ducts may be present. In one case, clustered, small, round calcifications on a screening mammogram proved to be in a nonpalpable 9 × 7 mm well-circumscribed, hypoechoic AME on sonography.37 Mammographically inapparent AMEs that were detected by sonography were described by Lee et al.38 and Chang et al.36 Adjacent duct ectasia is also a common finding. MRI studies are limited and show homogeneous to heterogeneous enhancement with a delayed washout pattern after gadolinium injection.31,38
Associated Tumors
Usual types of breast carcinoma can be present separately in a breast that harbors an AME. One of the patients studied by Lee et al.38 was a 72-year-old woman with two AMEs and a separate focus of DCIS in one breast. Three cases of AME with DCIS have been reported.27,42,43 Kuroda et al.44 described a 66-year-old woman with a 3.5-cm invasive duct carcinoma that was adjacent to, but separate from a 0.8-cm AME. A tumor described by Honda and Iyama45 appears to have been a malignant AME that was invaded by coexisting E-cadherin-negative invasive lobular carcinoma. Metastatic lobular carcinoma was present in axillary lymph nodes. Two years later, a nodule removed from a lung had the histologic appearance of the original AME, and similar lesions were found in both lungs and kidneys 5 years after the initial diagnosis. The biphasic structure of glandular cells surrounded by MECs that was present in the initial AME was also found in the metastatic foci. Da Silva et al.46 encountered a 0.5-cm AME adjacent to an adenoid cystic carcinoma (AdCC) in an area of tubular adenosis. Another patient reportedly had a breast mass composed of AME and recurrent phyllodes tumor.47
Gross Pathology
The gross size of AMEs ranges from 0.5 to 8.0 cm, with an average and median size of about 2.5 cm. With rare exceptions, the tumors have been described as solid, well circumscribed, and firm or hard; lobulation is often noted (Fig. 6.6). Several lesions were described as translucent. The color of the tumor cut surface has been characterized as tan, gray, white, yellow, and pink. Small cysts occur in a minority of cases.21,22,48 Very large, grossly cystic tumors can occupy a substantial part of the breast (Fig. 6.7). Some tumors have been described as predominantly intracystic.38,49 There does not appear to be a good correlation between the gross appearance of the tumor and its microscopic composition.
Microscopic Pathology
Benign AME
At low magnification, most AMEs are circumscribed and are composed of aggregated nodules, typically lacking a discrete fibrous capsule. Some AMEs consist of a compact nodular proliferation of epithelial cells and MECs, but most lesions are composed of solid or papillary nodules that often surround a slightly ovoid sclerotic center, in a configuration reminiscent of the petals of a flower (Fig. 6.8). A minority of AMEs consist, for the most part, of intraductal papillary elements, in accordance with the hypothesis that these lesions are variants of intraductal papilloma characterized by myoepithelial expansion (Fig. 6.9). Sometimes the papillary intraductal component extends into ducts outside the main body of the lesion. This characteristic may account for recurrence after a seemingly adequate excision. A minority of AMEs appear to arise from a lobular proliferation or adenosis (Figs. 6.10 and 6.11). Clear
cell epithelioid myoepithelial hyperplasia in an AME with the adenosis pattern could be mistaken for invasive carcinoma in a small biopsy sample (Fig. 6.11). The most unusual and exceedingly rare adenosis variant of AME has an infiltrative growth pattern that resembles MGA (Fig. 6.12). However, true MGA is characterized by the absence of myoepithelium (see discussion of MGA in Chapter 7).
cell epithelioid myoepithelial hyperplasia in an AME with the adenosis pattern could be mistaken for invasive carcinoma in a small biopsy sample (Fig. 6.11). The most unusual and exceedingly rare adenosis variant of AME has an infiltrative growth pattern that resembles MGA (Fig. 6.12). However, true MGA is characterized by the absence of myoepithelium (see discussion of MGA in Chapter 7).
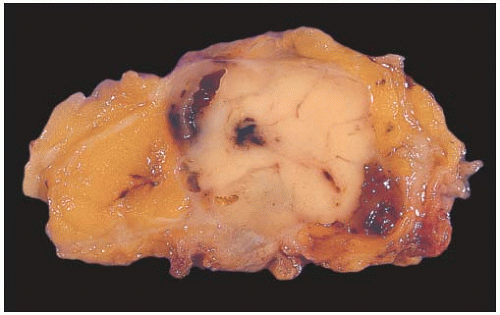 FIG. 6.6. Adenomyoepithelioma. This bisected tumor is circumscribed and has a nodular architecture. The tumor measures about 2 cm. |
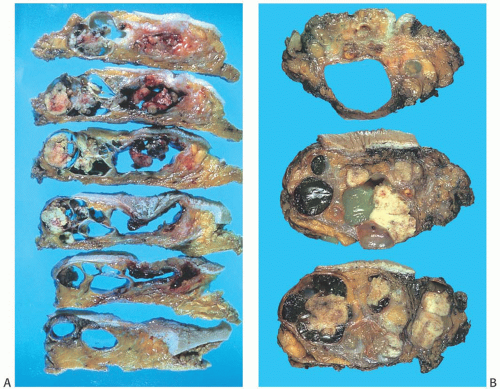 FIG. 6.7. Adenomyoepithelioma. Two different gross specimens are illustrated. Skin is present on the upper borders of the specimens. A: Multiple transverse sections of a mastectomy extensively involved by a multicystic AME with papillary areas. B: A solid and cystic multinodular AME that measured about 7 cm in greatest diameter is shown in transverse sections. |
The basic structural unit of the typical AME is centered on a small round or oval glandular lumen encompassed by cuboidal epithelial cells. The glands are surrounded by polygonal or spindle-shaped MECs with clear cytoplasm, bounded in turn by basement membrane (Fig. 6.13). The tubular type of AME, the most common microscopic pattern, features a balanced proliferation of round, oval, or tubular glandular elements invested by polygonal MECs with clear cytoplasm. In some tumors, MECs with clear cytoplasm are more numerous than epithelial cells and compress the tubular lumens, resulting in zones virtually devoid of glands (Fig. 6.14). Other lesions feature MECs proliferating in broad bands and trabeculae separated by strands of basement membrane or stroma (Figs. 6.15 and 6.16). The contrast between the dark-staining cytoplasm of the glandular cells and the pale or pink cytoplasm of MECs can be striking and provides a helpful clue to the diagnosis (Fig. 6.17). Small glandular lumens formed within the epithelial areas may have a pattern reminiscent of an endocrine neoplasm. In papillary regions, distinct polygonal MECs accompany the epithelium in its various branches and ramifications.
Metaplasia in AME
The epithelial cells tend to have sparse, darkly stained cytoplasm and hyperchromatic nuclei. In some cases, this produces a plasmacytoid appearance. Apocrine metaplasia may
be encountered in the glandular epithelium,16 particularly in papillary areas (Fig. 6.18). Glands exhibiting sebaceous metaplasia (Fig. 6.19) can occur focally in a minority of cases. Squamous metaplasia (Fig. 6.20) can also occur and sometimes can be florid, raising the differential diagnosis of squamous carcinoma. Cartilaginous metaplasia of the stroma is rarely seen (Fig. 6.21). A previously undescribed AME with mucoepidermoid differentiation has been encountered (Fig. 6.22).
be encountered in the glandular epithelium,16 particularly in papillary areas (Fig. 6.18). Glands exhibiting sebaceous metaplasia (Fig. 6.19) can occur focally in a minority of cases. Squamous metaplasia (Fig. 6.20) can also occur and sometimes can be florid, raising the differential diagnosis of squamous carcinoma. Cartilaginous metaplasia of the stroma is rarely seen (Fig. 6.21). A previously undescribed AME with mucoepidermoid differentiation has been encountered (Fig. 6.22).
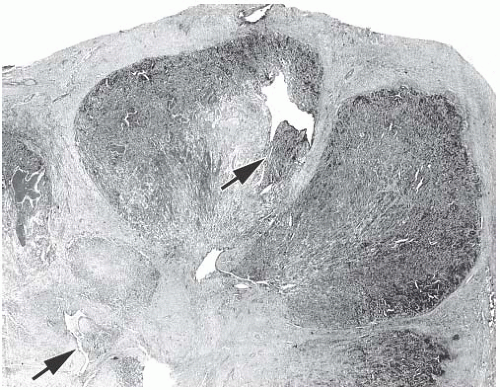 FIG. 6.8. Adenomyoepithelioma. A whole-mount histologic section in which multiple nodules surround a sclerotic core. There are remnants of ducts with papillary elements (arrows). |
Cytologic atypia and prominent mitotic activity are very infrequent or absent from lesions in which the MECs retain a polygonal configuration, and there are conspicuous papillary areas. Infrequent mitoses can be found in both epithelial and myoepithelial components. Stromal and myoepithelial elements may have an adenoid cystic pattern. This may be manifested by the presence of collagenous spherulosis29 (Fig. 6.15). Calcifications are rarely present, but some tumors develop central fibrosis or ischemic necrosis leading to calcification (Fig. 6.23). Squamous metaplasia is common in an infarcted AME (Fig. 6.24). The cystic papillary type of AME is very uncommon (Fig. 6.25).
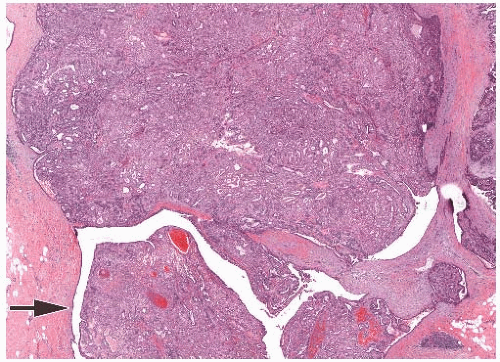 FIG. 6.9. Adenomyoepithelioma with papillary area. An AME with predominantly solid growth (top half) has an area with papillary architecture (arrow). |
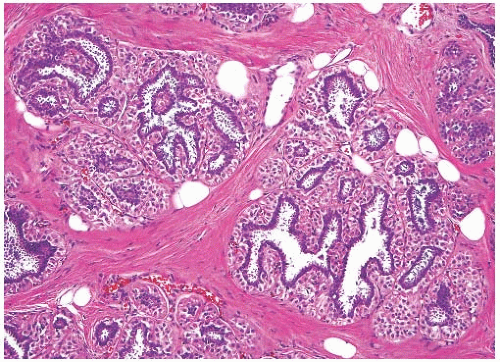 FIG. 6.10. Adenomyoepithelial hyperplasia of lobules. A group of lobules in which hyperplastic MECs surround the lobular glands, duplicating the pattern found in an AME. |
Spindle Cells in AMEs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree