and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
13.1 Definition, Site and Incidence
Myoepithelial and epithelial-myoepithelial carcinoma are two tumour entities that consist largely of malignant myoepithelial cells. Myoepithelial carcinoma (malignant myoepithelioma) (MC) consists almost entirely of myoepithelial cells with only the occasional duct-like structure. It was included first in the second WHO classification of 1991 and defined as ‘a rare malignant epithelial tumour composed of atypical myoepithelial cells with increased mitotic activity and aggressive growth’ [51].
Epithelial-myoepithelial carcinoma (EMC), on the other hand, is a tumour with biphasic histology. It is defined as ‘a tumour composed of variable proportions of two cell types which typically form duct-like structures. There is an inner layer of duct-lining cells and an outer layer of clear cells’ [52]. The outer clear cells are myoepithelial differentiated cells.
13.1.1 Myoepithelial Carcinoma
Myoepithelial carcinoma (MC) consists almost entirely of modified myoepithelial cells. A malignant salivary gland tumour consisting of myoepithelial cells but containing more than the occasional true luminal cells should not be classified as an MC. This definition is in line with that separating its benign counterpart, the myoepithelioma, from pleomorphic adenoma. MC is distinguished from myoepithelioma by its infiltrative, destructive growth. MC is a rare tumour, and in 1996 Ellis and Auclair found only 35 cases from the English literature and the AFIP register. Furthermore, they note that MC only constituted 0.2 % of all salivary gland neoplasms in their register during a 10-year period [14]. Although MC is a quite rare tumour, clinicopathological studies during the last 15 years indicate that it may not be as extremely rare as considered previously. The largest series before 1996 was that reported in 1993 by Di Palma and Guzzo and that consisted of ten cases [10]. In their review of the literature, Di Palma and Guzzo found less than 100 reported cases of myoepithelial tumours, the vast majority of which were benign or locally aggressive and very few had overt malignant features. In fact, less than 20 cases were reported as MC before 1993 [8, 10, 11, 24, 28, 56, 60, 61, 63]. This paucity is partly due to the fact that in the older literature many cases of MC were likely reported as carcinoma ex pleomorphic adenoma rather than subclassified as myoepithelial carcinoma. MC is nevertheless a rare salivary malignancy and is today considered to represent less than 2 % of salivary gland carcinomas [57].
MC is predominantly a parotid tumour. The majority of cases, 60–70 %, have arisen in the parotid gland. The minor salivary glands are the primary site in 25 % of cases, whilst approximately 10 % arise in the submandibular gland [9, 10, 14, 26, 39, 47, 68]. MC arises de novo or from a pre-existing benign tumour, most commonly from pleomorphic adenoma but also from myoepithelioma. In 14 of the 25 cases of MC reported by Savera and associates [47], there was unequivocal histological evidence of underlying benign tumour. Twelve of these were pleomorphic adenoma and two were myoepithelioma. Similarly, Di Palma and Guzzo [10] reported five of their ten cases to have arisen in a pre-existing pleomorphic adenoma and five de novo. The study by Alós and associates [1] comprised 16 myoepithelial neoplasms, four of which were MC and all of which had developed from pre-existing pleomorphic adenoma and myoepithelioma. From the literature it hence appears that MC arises more frequently from a pre-existing benign salivary gland tumour than de novo. As for most intraoral salivary gland tumours, its benign counterpart myoepithelioma inclusive, the palate is the most common intraoral site for MC.
Myoepithelial tumours arising in minor salivary glands outside the oral cavity is an exceedingly rare event, but MC has been reported in the larynx [20, 28], nasopharynx [41, 64], lungs [35] and even a rare case in the infratemporal fossa [21]. Myoepithelial carcinoma may also arise in the skin and soft tissue [3, 25, 36, 59].
Due to its rarity and relatively small series studied, little information on the clinical signs and symptoms of MC has been obtained. The largest study so far comprised 25 patients, and in most of those cases, the tumour presented as a painless mass [47]. MC may be locally destructive and the tumour size varies usually being between 2 and 10 cm, although huge tumours up to 20 cm have been described. MCs are devoid of a true capsulate but can be well circumscribed and usually have a grey-whitish cut surface, occasionally with necrosis and cystic degeneration. In contrast to the slight female predilection observed for myoepithelioma, males and females appear to be affected equally. The mean age of presentation is the sixth decade but shows a very wide age distribution (range 14–86 years) [1, 10, 14, 47].
13.1.2 Epithelial-Myoepithelial Carcinoma
Epithelial-myoepithelial carcinoma (EMC) is a tumour consisting of various proportions of two cell types which typically form duct-like structures. There is an inner layer of duct-lining cells and an outer layer of clear myoepithelial cells [52]. The clear cells are often relatively large and usually predominate. The term epithelial-myoepithelial carcinoma was introduced in 1972 by Donath and associates [13] and later firmly established after the report of 16 cases in 1982 by Corio and associates [7]. EMC was recognised as a separate entity in the 2nd WHO classification in 1991 [52] but had before that been described under various names, e.g. glycogen-rich adenoma [23], glycogen-rich adenocarcinoma [38], clear cell adenoma [45] and clear cell carcinoma [5].
EMC is like myoepithelial carcinoma, a rare salivary malignancy and also constitutes 1–2 % of salivary carcinomas [15, 17]. Most publications are case reports [27, 29, 31, 42, 62, 66], but also a smaller number of clinicopathological studies have been published during the last two decades [6, 40, 43, 53]. The clinicopathological features and immunophenotypic characteristics of 61 cases of EMC were reported by Seethala and associates [49], which is the largest series of epithelial-myoepithelial carcinomas published so far.
13.2 Histopathology
13.2.1 Myoepithelial Carcinoma
Grossly, myoepithelial carcinomas (MC) are unencapsulated but may be well defined with nodular surfaces. The cut surface is grey-white and can be glassy. Some tumours reveal areas of haemorrhage, necrosis and pseudocystic degeneration.
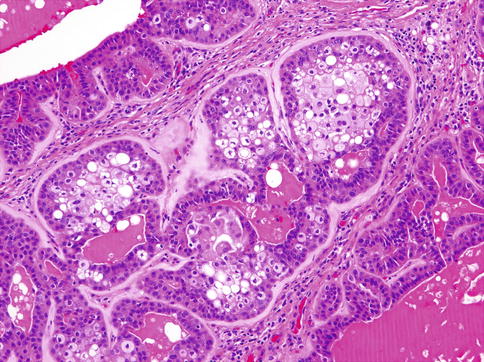
Histologically, MCs are characterised by multilobulated growth pattern, not infrequently with necrotic foci, with infiltration into adjacent tissues (Fig. 13.1). Perineural and vascular invasion is seen in 44 and 16 %, respectively. The tumour-related extracellular matrix is generally prominent and is myxoid or hyalinised. There is a considerable range of microscopic features both in architecture and cytology, reflecting the different forms of modified myoepithelial cells. The nuclei of MC may be relatively uniform, small to intermediate sized and composed of finely distributed chromatin, lacking obvious nucleoli, or there may be marked cytological atypia, with enlarged pleomorphic nuclei, showing chromatin clumping and large nucleoli. Multinucleated and bizarre tumour giant cells may occasionally be present.
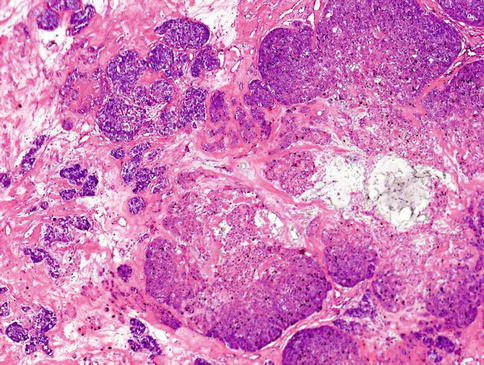

Fig. 13.1
MC is characterised by multilobulated growth pattern, not infrequently with necrotic foci, with infiltration into adjacent tissues
The neoplastic nodules comprise solid, reticular and sheet-like growth patterns, often with plentiful myxoid (Fig. 13.2) and hyaline extracellular matrix (Fig. 13.3). The range of cell types includes epithelioid cells often arranged in trabecular and pseudoacinar and duct-like cleft formations within abundant myxoid stroma (Fig. 13.4), cells with clear cytoplasm sometimes lipoblast-like, and sometimes appearing signet ring-like, hyaline-plasmacytoid and spindle-shaped cells (Fig. 13.5). In most MCs, one cell type predominates, but there is usually a minority of other cell types, too. Metaplastic changes are frequently seen and include squamous, chondroid and sebaceous features. Mitotic figures may be scanty to plentiful and include atypical forms. A unique case of MC of minor salivary gland with numerous collagenous crystalloids has been reported [4].
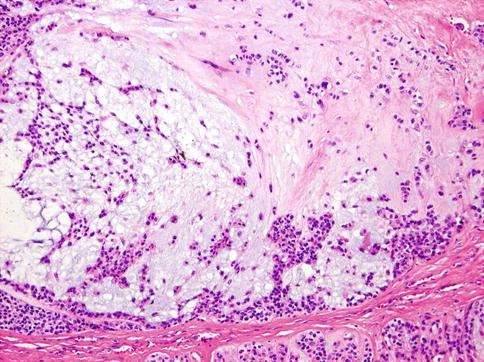
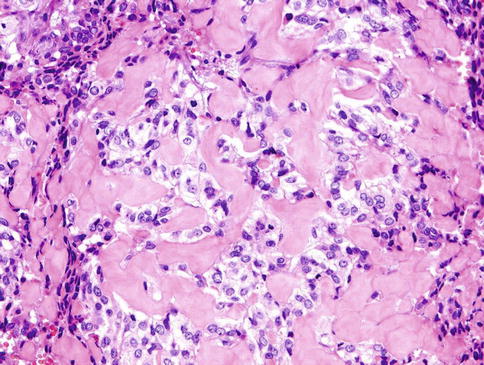
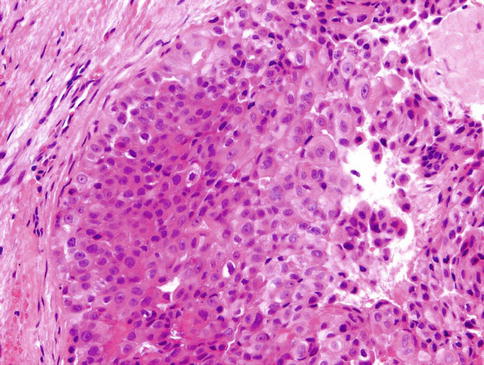
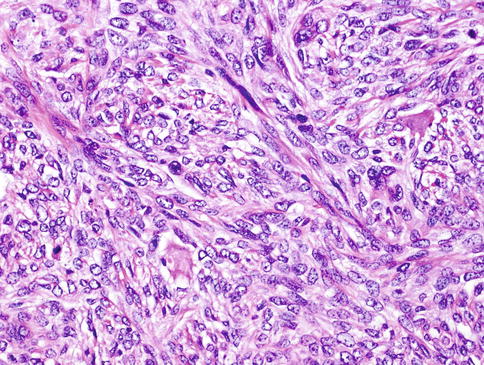

Fig. 13.2
The neoplastic nodules comprise solid, reticular and sheet-like growth patterns, often with plentiful myxoid

Fig. 13.3
Hyaline extracellular matrix

Fig. 13.4
The range of cell types includes epithelioid cells often arranged in solid and pseudoacinar patterns

Fig. 13.5
Spindle-shaped neoplastic myoepithelial cells
13.2.1.1 Clear Cell Variant of Myoepithelial Carcinoma
Clear cell MC is composed of solid nodules separated by thin fibrous septa and made of compact nests of polyhedral cells with abundant clear cytoplasm and round, sometimes vesicular nuclei with well-defined boundaries (Fig. 13.6). Only small quantities of glycogen could be demonstrated in the cytoplasm. In places, multilayering of cells in the nests mimics the double lining of ducts in epithelial-myoepithelial carcinoma, but the cells in MC are all of the same type. Patchy squamous metaplasia is often present (Fig. 13.7), and very occasional small tubular structures can sometimes be identified. A very prominent feature is an accumulation of alcian blue-positive, mucicarmine-negative, mesenchymal mucinous matrix within which the tumour cells are placed. Occasionally, mucinous matrix predominates to form cystic spaces in which the neoplastic cells appear to float. Comedo-like necrotic foci are often seen (Fig. 13.8). A very characteristic feature is the presence of PAS-positive hyaline deposits of basement membrane-like extracellular matrix material. These structures vary in size from thickened lamellae dividing cellular lobules to small hyaline droplets (Fig. 13.9). The latter sometimes attain a greater size, so that they form collagenous spherules [33, 37].
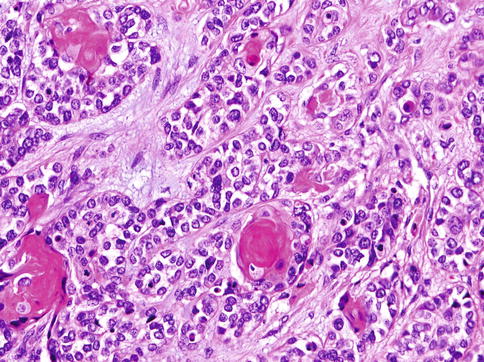
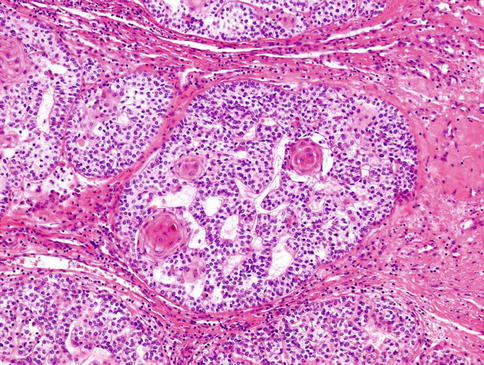
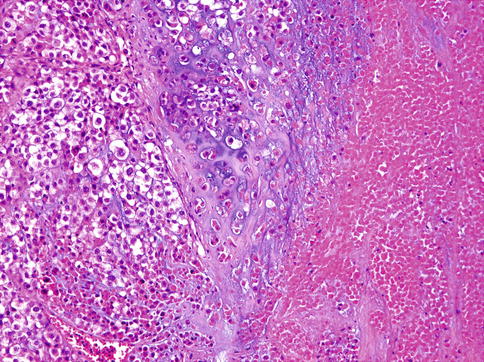
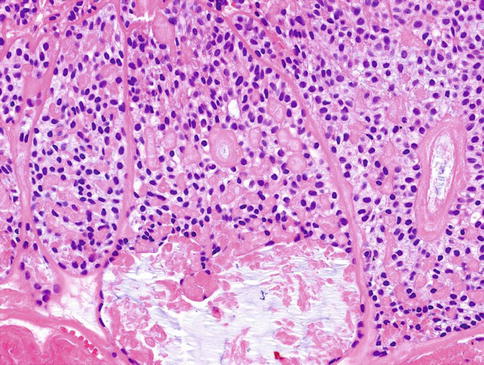

Fig. 13.6
Clear cell MC is composed of solid nodules separated by thin fibrous septa and made of compact nests of polyhedral cells with abundant clear cytoplasm. The cells have round, sometimes vesicular nuclei with well-defined boundaries

Fig. 13.7
Patchy squamous metaplasia is often present in clear cell MC

Fig. 13.8
Comedo-like necrotic foci can be present in clear cell MC

Fig. 13.9
Extracellular matrix deposits vary from thickened lamellae dividing cellular lobules to small hyaline droplets
No true glandular and tubular structures are present in myoepithelial carcinomas, but as with their benign counterparts, occasional small ducts in a neoplasm with otherwise typical features should not preclude the diagnosis [55].
13.2.2 Epithelial-Myoepithelial Carcinoma (EMC)
Grossly, the majority of tumours display a multinodular growth pattern, pushing borders, and are partially to completely encapsulated. The infiltrative growth pattern of EMC is less common, observed in about 12 % [49].
13.2.2.1 Classic Variant of EMC
Classic variant of EMC consists of a bilayered arrangement of tubules with inner ductal cells and abluminal myoepithelial cells (Fig. 13.10). All EMCs are characterised by areas of classic biphasic tubular/trabecular patterns with an outer layer of clear myoepithelial cells and inner layer of epithelial cells. A significant proportion of the tumours show at least focal solid growth pattern which is generally myoepithelial and only rarely epithelial [49]. The tumour nests are accompanied by varying degrees of hyaline sclerosis. Ductal structures may form papillary and cystic pattern and solid component consisting of clear myoepithelial cells. In rare cases, proliferation of spindle cells and squamous metaplasia can be noted. In the myoepithelial component, the cytoplasm ranges from clear to pale amphophilic (Fig. 13.11).
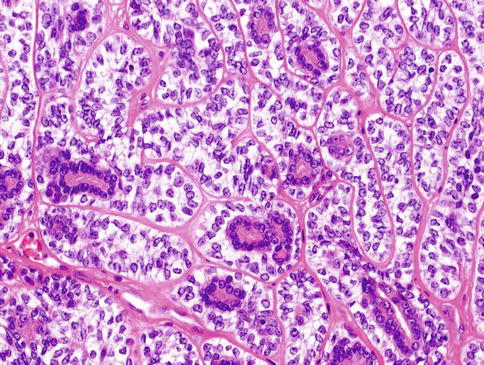
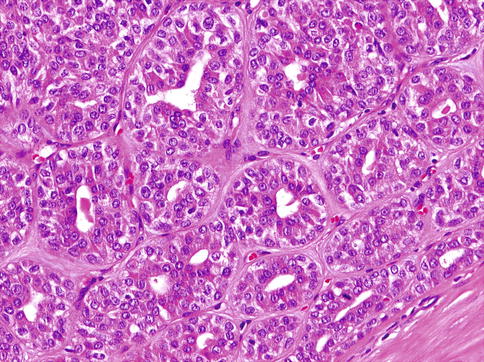

Fig. 13.10
Classic variant of EMC consists of a bilayered arrangement of tubules with inner ductal cells and abluminal myoepithelial cells

Fig. 13.11
Myoepithelial cells range from clear to pale amphophilic
EMC has several described variants which result from various combinations of unusual growth patterns, nuclear features and/or cytoplasmic characteristics. These include EMC with high-grade transformation, EMC ex pleomorphic adenoma, double clear EMC, sebaceous EMC, oncocytic EMC and apocrine EMC [49]. In addition, the modified neoplastic myoepithelial cells in EMC may show alterations such as ‘ancient change’ or spindled morphology (Fig. 13.12) or with Verocay-like palisading or squamous metaplasia [43, 49, 50].
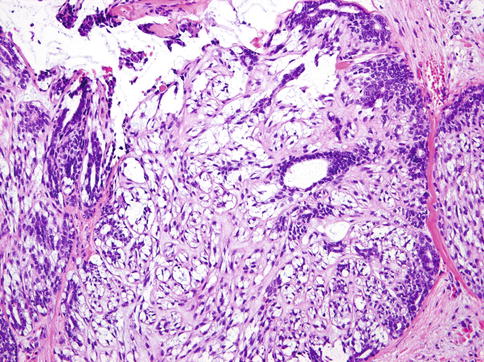

Fig. 13.12
EMC with modified spindle-shaped myoepithelial cells with clear cytoplasm
EMC with high-grade transformation encompasses tumours designated historically as dedifferentiated EMC and EMC with anaplasia [2, 18, 30, 32, 43, 49, 53]. High-grade transformation (dedifferentiation) is defined as the histological progression of a low-grade malignant neoplasm to a high-grade neoplasm in which the original line of differentiation is no longer evident. It can occur at the time of initial presentation or at recurrence. The phenomenon has been recognised in a variety of salivary gland carcinomas, such as acinic cell carcinoma, mammary analogue secretory carcinoma, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma and epithelial-myoepithelial carcinoma (see Chap. 16). The high-grade tumours within the spectrum of EMC comprised about 7 % of cases in one series [49]. Histologically, EMC with high-grade transformation is composed of two clearly separated distinct carcinomatous components; one component has features of a low-grade classic EMC, and the other component displays features of a high-grade carcinoma. Mitotic figures are extremely rare, nuclear atypia is mild, and necrosis is absent in the low-grade EMC component. In comparison, the high-grade component EMC consists of solid and sheet-like growth patterns with marked central comedonecrosis; it often has infiltrative growth pattern and skeletal muscle and skin invasion [32]. The nuclei are large pleomorphic with conspicuous nucleoli, and a high mitotic rate is seen. Also, bizarre tumour cells can be observed occasionally. The high-grade component lacks features of biphasic glandular structures typical of classic EMC.
13.2.2.2 Oncocytic and Apocrine Variant of EMC
Oncocytic variant of EMC is composed of tubules larger in calibre, more akin to striated ducts than intercalated ducts. The ductal component in oncocytic EMC consists of cuboidal to columnar oncocytic cells with granular cytoplasm, reminiscent of striated duct epithelium (Fig. 13.13). The outer myoepithelial cell layer varies and ranges from clear to oncocytic. Nuclei of the myoepithelial component are larger and more vesicular than the ductal component, similar to classic EMC. When the myoepithelial component is oncocytic, the bilayered appearance may be difficult to discern. Oncocytic EMC has a propensity to show a bilayered papillary growth pattern and luminal calcifications. Oncocytic EMC was described initially by Savera and Salama in 2005 [46], and apocrine EMC was described by Seethala and associates [50]. Whilst oncocytic and apocrine changes may be seen focally in many otherwise conventional classic EMC, oncocytic and apocrine EMC are only designated as variants when the oncocytic or apocrine components comprise greater than 50 % of the tumour area. Both variants are unique in that they are ‘pink’ or oncocytoid in appearance, challenging the classical requirement for clear cell morphology in the myoepithelial component of EMC [48].
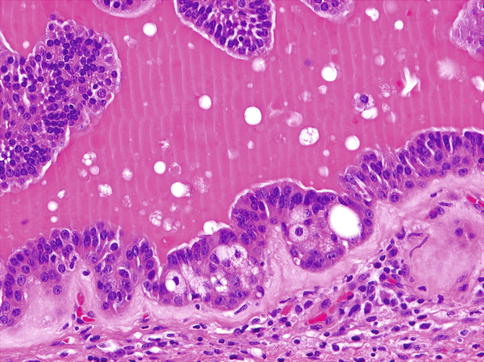

Fig. 13.13
Oncocytic EMC consists of cuboidal to columnar oncocytic cells with granular cytoplasm, reminiscent of striated duct epithelium and outer clear myoepithelial layer
13.2.2.3 Sebaceous Variant of EMC
Focal sebaceous differentiation in EMC was first mentioned in one recent large study in 13 % of cases [49]; however, the first full description of sebaceous variant of EMC should be ascribed to Shinozaki and associates [53]. Histologically sebaceous EMC is composed of foci of sebaceous differentiation admixed with bilayered component of classic EMC. Bilayered duct-like structures are composed of an inner layer of duct-lining epithelial cells and an outer layer of clear myoepithelial-type cells. The sebaceous component is either distributed diffusely or focally with clusters of sebaceous cells observed in the centre of the solid nests surrounded by myoepithelial cells (Fig. 13.14). The tumour cells with sebaceous differentiation are relatively large and exhibit multiple, small and round vacuoles in their cytoplasm. The tumour cells with totally vacuolated cytoplasm and peripherally located nuclei, superficially resembling lipoblasts, can be encountered [50, 53].