INTRODUCTION
The mycobacteria are rod-shaped, aerobic bacteria that do not form spores. Although they do not stain readily, after being stained, they resist decolorization by acid or alcohol and are therefore called “acid-fast” bacilli. Mycobacterium tuberculosis causes tuberculosis and is a very important pathogen of humans. Mycobacterium leprae causes leprosy. Mycobacterium avium-intracellulare (M avium complex, or MAC) and other nontuberculous mycobacteria (NTM) frequently infect patients with AIDS, are opportunistic pathogens in other immunocompromised persons, and occasionally cause disease in patients with normal immune systems. There are more than 200 Mycobacterium species, including many that are saprophytes. The mycobacteria that infect humans are listed in Table 23-1.
| Species | Reservoir | Common Clinical Manifestations; Comment |
|---|---|---|
| SPECIES ALWAYS CONSIDERED PATHOGENS | ||
Mycobacterium tuberculosis Mycobacterium leprae Mycobacterium bovis | Humans Humans Humans, cattle | Pulmonary and disseminated tuberculosis; millions of cases annually in the world Leprosy Tuberculosis-like disease; rare in North America; M bovis is closely related to M tuberculosis |
| SPECIES POTENTIALLY PATHOGENIC IN HUMANS | ||
| Moderately common causes of disease | ||
| Mycobacterium avium complex | Soil, water, birds, fowl, swine, cattle, environment | Disseminated, pulmonary; very common in untreated AIDS patients; occurs in other immunosuppressed patients; uncommon in patients with normal immune systems |
| Mycobacterium kansasii | Water, cattle | Pulmonary, other sites |
| Uncommon to very rare causes of disease | ||
| Mycobacterium africanum | Humans, monkeys | Pulmonary cultures; resembles M tuberculosis; rare |
| Mycobacterium genavense | Humans, pet birds | Blood in AIDS patients; grows in liquid medium (BACTEC) and on solid medium supplemented with mycobactin J; grows in 2–8 weeks |
| Mycobacterium haemophilum | Unknown | Subcutaneous nodules and ulcers primarily in AIDS patients; requires hemoglobin or hemin; grows at 28°–32°C; rare |
| Mycobacterium malmoense | Unknown, environment | Pulmonary, tuberculosis-like (adults), lymph nodes (children); most reported cases are from Sweden, but organism may be much more widespread; M malmoense is closely related to Mycobacterium avium-intracellulare; takes 8–12 weeks to grow |
| Mycobacterium marinum | Fish, water | Subcutaneous nodules and abscesses, skin ulcers |
| Mycobacterium scrofulaceum | Soil, water, moist foods | Cervical lymphadenitis; usually cured by incision, drainage, and removal of involved lymph nodes |
| Mycobacterium nonchromogenicum | Environment | The primary pathogen in the Mycobacterium terrae complex. Causes tenosynovitis of the hand |
Mycobacterium simiae (new members of M simiae complex include M lentiflavum, M triplex, M europaeum) | Monkeys, water | Pulmonary, disseminated in AIDS patients; rare |
| Mycobacterium szulgai | Unknown | Pulmonary, tuberculosis-like; rare |
| Mycobacterium ulcerans | Humans, environment | Subcutaneous nodules and ulcers; may be severe; M ulcerans is closely related to M marinum; takes 6–12 weeks to grow; optimal growth at 33°C suggests environmental source; rare |
| Mycobacterium xenopi | Water, birds | Pulmonary, tuberculosis-like with preexisting lung disease; rare |
| Rapid growers | ||
| Mycobacterium abscessus | Soil, water, animals | Most frequently isolated rapid grower from pulmonary infections; skin and soft tissue infections; frequently multidrug resistant |
| Mycobacterium chelonae | Soil, water, animals, marine life | Cutaneous lesions most common, subcutaneous abscesses, disseminated infections in immunocompromised patients |
| Mycobacterium fortuitum | Soil, water, animals | Consists of a complex of organisms that can only be differentiated by molecular methods; associated with nail salon furunculosis; pulmonary infections similar to M abscessus |
| Mycobacterium immunogenum | Environment | Associated with pseudo-outbreaks linked to contaminated equipment in hospitals; isolates have been associated with joint disease, skin ulcers, catheter infections, and some pulmonary disease; closely related to M chelonae-abscessus |
| Mycobacterium mucogenicum | Unknown | CVC-associated infections are the most important infections associated with this organism; name reflects its mucoid appearance in culture |
| SAPROPHYTIC SPECIES THAT VERY RARELY CAUSE DISEASE IN HUMANS | ||
Mycobacterium gordonae Mycobacterium flavescens Mycobacterium fallax Mycobacterium gastri Mycobacterium smegmatis Mycobacterium terrae complex | Water Soil, water Soil, water Gastric washings Soil, water Soil, water | These saprophytic Mycobacterium species are very uncommon causes of disease in humans; positive culture results for these mycobacteria usually represent environmental contamination of specimens and not disease; many of the saprophytic mycobacteria grow best at temperatures <33°C; there are many other saprophytic Mycobacterium species not listed here that seldom, if ever, appear in cultures of patients’ specimens |
MYCOBACTERIUM TUBERCULOSIS
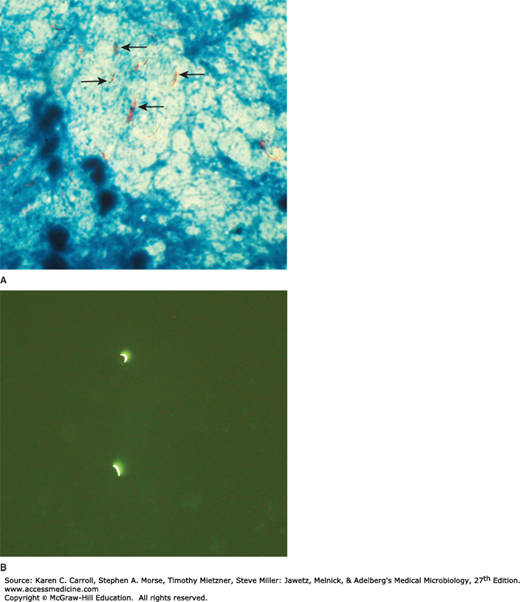
In tissue, tubercle bacilli are thin, straight rods measuring about 0.4 × 3 μm (Figure 23-1). On artificial media, coccoid and filamentous forms are seen with variable morphology from one species to another. Mycobacteria cannot be classified as either gram positive or gram negative. When stained by basic dyes, they cannot be decolorized by alcohol, regardless of treatment with iodine. True tubercle bacilli are characterized by “acid fastness”—that is, 95% ethyl alcohol containing 3% hydrochloric acid (acid-alcohol) quickly decolorizes all bacteria except the mycobacteria. Acid fastness depends on the integrity of the waxy envelope. The Ziehl-Neelsen technique of staining is used for identification of acid-fast bacteria. The method is detailed in Chapter 47. In smears of sputum or sections of tissue, mycobacteria can be demonstrated by yellow-orange fluorescence after staining with fluorochrome stains (eg, auramine, rhodamine). The ease with which acid-fast bacteria can be visualized with fluorochrome stains makes them the preferred stains for clinical specimens (Figure 23-1B). The availability of ultrabright light-emitting diode microscopes, some of which do not require electricity, has advanced fluorescence microscopy in resource-limited countries.
FIGURE 23-1
A: Mycobacterium tuberculosis (arrows) in a processed sputum specimen stained by Ziehl-Neelsen stain. The Mycobacterium tuberculosis is red against a blue background. B: The fluorescent dye Auramine O was used to stain a sputum sample. It shows two fluorescent M tuberculosis. Original magnification ×1000. (Courtesy of G Cunningham.)
The media for primary culture of mycobacteria should include a nonselective medium and a selective medium. Selective media contain antibiotics to prevent the overgrowth of contaminating bacteria and fungi. There are three general formulations that can be used for both the nonselective and selective media. Agar-based (solid) media are useful for observing colony morphology, for detection of mixed cultures, for antimicrobial susceptibility testing, and can also provide some indication of the quantity of organisms in a particular specimen.
1. Semisynthetic agar media—These media (eg, Middle-brook 7H10 and 7H11) contain defined salts, vitamins, cofactors, oleic acid, albumin, catalase, and glycerol; the 7H11 medium also contains casein hydrolysate. The albumin neutralizes the toxic and inhibitory effects of fatty acids in the specimen or medium. Large inocula yield growth on these media in several weeks. Because large inocula may be necessary, these media may be less sensitive than other media for primary isolation of mycobacteria.
2. Inspissated egg media—These media (eg, Löwenstein-Jensen) contain defined salts, glycerol, and complex organic substances (eg, fresh eggs or egg yolks, potato flour, and other ingredients in various combinations). Malachite green is included to inhibit other bacteria. Small inocula in specimens from patients will grow on these media in 3–6 weeks.
These media with added antibiotics (Gruft and Mycobactosel) are used as selective media.
3. Broth media—Broth media (eg, Middlebrook 7H9 and 7H12) support the proliferation of small inocula. Ordinarily, mycobacteria grow in clumps or masses because of the hydrophobic character of the cell surface. If tweens (water-soluble esters of fatty acids) are added, they wet the surface and thus permit dispersed growth in liquid media. Growth is often more rapid than on complex media. There are several commercial sources of these media that are used in many clinical and reference laboratories. These include the MGIT system (Becton Dickinson, Sparks, MD), VersaTREK® Culture System (ThermoFisher Scientific, Houston, TX), and MB Redox (Heipha Diagnostica Biotest, Eppelheim, Germany).
Mycobacteria are obligate aerobes and derive energy from the oxidation of many simple carbon compounds. Increased CO2 tension enhances growth. Biochemical activities are not characteristic, and the growth rate is much slower than that of most bacteria. The doubling time of tubercle bacilli is about 18 hours. Saprophytic forms tend to grow more rapidly, to proliferate well at 22–33°C, to produce more pigment, and to be less acid fast than pathogenic forms.
Mycobacteria tend to be more resistant to chemical agents than other bacteria because of the hydrophobic nature of the cell surface and their clumped growth. Dyes (eg, malachite green) or antibacterial agents (eg, penicillin) that are bacteriostatic to other bacteria can be incorporated into media without inhibiting the growth of tubercle bacilli. Acids and alkalies permit the survival of some exposed tubercle bacilli and are used to help eliminate contaminating organisms and for “concentration” of clinical specimens. Tubercle bacilli are resistant to drying and survive for long periods in dried sputum.
Variation can occur in colony appearance, pigmentation, virulence, optimal growth temperature, and many other cellular or growth characteristics.
There are marked differences in the ability of different mycobacteria to cause lesions in various host species. Humans and guinea pigs are highly susceptible to M tuberculosis infection, but fowl and cattle are resistant. M tuberculosis and Mycobacterium bovis are equally pathogenic for humans. The route of infection (respiratory vs intestinal) determines the pattern of lesions. In developed countries, M bovis has become very rare. Some “atypical” mycobacteria, now designated as NTM (eg, Mycobacterium kansasii), produce human disease indistinguishable from tuberculosis; others (eg, Mycobacterium fortuitum) cause only surface lesions or act as opportunists.
The constituents listed as follows are found mainly in cell walls. Mycobacterial cell walls can induce delayed hypersensitivity and some resistance to infection and can replace whole mycobacterial cells in Freund’s adjuvant. Mycobacterial cell contents only elicit delayed hypersensitivity reactions in previously sensitized animals.
Mycobacteria are rich in lipids. These include mycolic acids (long-chain fatty acids C78–C90), waxes, and phosphatides. In the cell, the lipids are largely bound to proteins and polysaccharides. Muramyl dipeptide (from peptidoglycan) complexed with mycolic acids can cause granuloma formation; phospholipids induce caseous necrosis. Lipids are to some extent responsible for acid fastness. Their removal with hot acid destroys acid fastness, which depends on both the integrity of the cell wall and the presence of certain lipids. Acid fastness is also lost after sonication of mycobacterial cells. Analysis of lipids by gas chromatography reveals patterns that aid in classification of different species.
Virulent strains of tubercle bacilli form microscopic “serpentine cords” in which acid-fast bacilli are arranged in parallel chains. Cord formation is correlated with virulence. A “cord factor” (trehalose-6,6′-dimycolate) has been extracted from virulent bacilli with petroleum ether. It inhibits migration of leukocytes, causes chronic granulomas, and can serve as an immunologic “adjuvant.”
Each type of mycobacterium contains several proteins that elicit the tuberculin reaction. Proteins bound to a wax fraction can, upon injection, induce tuberculin sensitivity. They can also elicit the formation of a variety of antibodies.
Mycobacteria contain a variety of polysaccharides. Their role in the pathogenesis of disease is uncertain. They can induce the immediate type of hypersensitivity and can serve as antigens in reactions with sera of infected persons.
Mycobacteria are emitted in droplets smaller than 25 μm in diameter when infected persons cough, sneeze, or speak. The droplets evaporate, leaving organisms that are small enough, when inhaled, to be deposited in alveoli. Inside the alveoli, the host’s immune system responds by release of cytokines and lymphokines that stimulate monocytes and macrophages. Mycobacteria begin to multiply within macrophages. Some of the macrophages develop an enhanced ability to kill the organism, but others may be killed by the bacilli. Pathogenic lesions associated with infection appear in the lung 1–2 months after exposure. Two types of lesions as described later under Pathology may develop. Resistance and hypersensitivity of the host greatly influence development of disease and the type of lesions that are seen.
The production and development of lesions and their healing or progression are determined chiefly by (1) the number of mycobacteria in the inoculum and their subsequent multiplication and (2) the type of host and immune response.
1. Exudative type—This consists of an acute inflammatory reaction with edema fluid; polymorphonuclear leukocytes; and, later, monocytes around the tubercle bacilli. This type is seen particularly in lung tissue, where it resembles bacterial pneumonia. It may heal by resolution so that the entire exudate becomes absorbed; it may lead to massive necrosis of tissue or may develop into the second (productive) type of lesion. During the exudative phase, the tuberculin test result becomes positive.
2. Productive (proliferative) type—When fully developed, this lesion, a chronic granuloma, consists of three zones: (1) a central area of large, multinucleated giant cells containing tubercle bacilli; (2) a mid zone of pale epithelioid cells, often arranged radially; and (3) a peripheral zone of fibroblasts, lymphocytes, and monocytes. Later, peripheral fibrous tissue develops, and the central area undergoes caseation necrosis. Such a lesion is called a tubercle. A caseous tubercle may break into a bronchus, empty its contents there, and form a cavity. It may subsequently heal by fibrosis or calcification.
Tubercle bacilli spread in the host by direct extension, through the lymphatic channels and bloodstream, and via the bronchi and gastrointestinal tract.
In the first infection, tubercle bacilli always spread from the initial site via the lymphatics to the regional lymph nodes. The bacilli may spread farther and reach the bloodstream, which in turn distributes bacilli to all organs (miliary distribution). The bloodstream can be invaded also by erosion of a vein by a caseating tubercle or lymph node. If a caseating lesion discharges its contents into a bronchus, they are aspirated and distributed to other parts of the lungs or are swallowed and passed into the stomach and intestines.
When mycobacteria establish themselves in tissue, they reside principally intracellularly in monocytes, reticuloendothelial cells, and giant cells. The intracellular location is one of the features that makes chemotherapy difficult and favors microbial persistence. Within the cells of immune animals, multiplication of tubercle bacilli is greatly inhibited.
When a host has first contact with tubercle bacilli, the following features are usually observed: (1) An acute exudative lesion develops and rapidly spreads to the lymphatics and regional lymph nodes. The exudative lesion in tissue often heals rapidly. (2) The lymph node undergoes massive caseation, which usually calcifies (Ghon lesion). (3) The tuberculin test result becomes positive.
As described in the early 20th century, primary infection type occurred usually in childhood, and involved any part of the lung but most often the mid-lung fields or the base. Enlarged hilar and mediastinal lymph nodes are frequently observed.
The reactivation type is usually caused by tubercle bacilli that have survived in the primary lesion. Reactivation tuberculosis is characterized by chronic tissue lesions, the formation of tubercles, caseation, and fibrosis. Regional lymph nodes are only slightly involved, and they do not caseate. The reactivation type almost always begins at the apex of the lung, where the oxygen tension (PO2) is highest.
These differences between primary infection and reinfection or reactivation are attributed to (1) resistance and (2) hypersensitivity induced by the first infection. It is not clear to what extent each of these components participates in the modified response in reactivation tuberculosis.
During the first infection with tubercle bacilli, a certain resistance is acquired, and there is an increased capacity to localize tubercle bacilli, retard their multiplication, limit their spread, and reduce lymphatic dissemination. This can be attributed to the development of cellular immunity, with evident ability of mononuclear phagocytes to limit the multiplication of ingested organisms and even to destroy them.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree